Biological question: Why aren’t proteins constantly disrupted by thermal fluctuations?
The cartoons in cell biology books show proteins snapping crisply between definite conformations, as they carry out their jobs. Can a floppy chain of residues really behave in this way?
Physical idea: Cooperativity sharpens the transitions of macromolecules and their assemblies.
As usual the section headings are informative.
9.1 Elasticity models of polymers
9.1.1 Why physics works (when it does work)
Here he gets to the heart of emergence and effective Hamiltonians introducing the important idea.
When we study a system with a large number of locally interacting, identical constituents, on a far bigger scale than the size of the constituents, then we reap a huge simplification: Just a few effective degrees of freedom describe the system’s behavior, with just a few phenomenological parameters.9.1.2 Four phenomenological parameters characterize the elasticity of a long, thin rod
9.1.3 Polymers resist stretching with an entropic force
9.2 Stretching single macromolecules
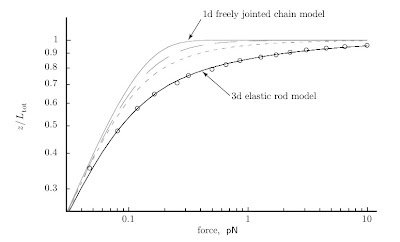
9.2.1 The force–extension curve can be measured for single DNA molecules
The lines are the predictions of a simple model for the length of a polymer versus the force applied to stretch it. The circles are actual experimental data for DNA molecules.
9.2.2 A simple two-state system qualitatively explains DNA stretching at low force




No comments:
Post a Comment