The apparent failure of resonance theory in these systems arises from the assumption that only low energy resonance forms need to be considered as contributing to the ground and first excited states.
Saturday, January 30, 2010
What determines the colour of an organic dye?
Friday, January 29, 2010
Beware of curve fitting
decreasing T moves subsequent temperature data sets up and to the right on the graph, even if the data themselves do not change with temperature at all. In fact, any weakly temperature dependent dataset that resembles a power-law can be made to fit onto a single line if plotted in this way with an appropriate choice of α. Data collapse with this plotting procedure is not sufficient to demonstrate TLL physics.
There is another reason why I am skeptical about any claim of "metalllic" behavior in these systems. Their mobility is much less than than the "minimum mobility"of 1 cm^2/Vsec that is necessary for the coherent transport associated with delocalised electrons and band structure.
Thursday, January 28, 2010
Examples of inhomogeneous mixed valence
Magnetite (Fe3O4) has the AM2X4 spinel structure, of the "inverse" type :
Magnetite has the empirical formula Fe3O4, or Fe2+(Fe3+O2)2, “ferrous ferrite”. Its formula as a spinel would be Fe3+tetFe2+octFe3+octO4 , where "tet" and "oct" stand for tetrahedral and octahedral coordinations by the oxide anions. In the above model, the blue spheres represent the tetrahedral iron(III) cations , and the red spheres are the octahedrally coordinated iron(II) and (III) cations. The oxide anions are shown as the green spheres. Because of the fortuitous inverse nature of the magnetite structure, ferrous and ferric cations are both in the similar octahedral coordination by oxides. In "normal" spinels, such as the mineral spinel itself (magnesium aluminate), the A cation is tetrahedral and the M cations are both octahedral:
Wednesday, January 27, 2010
Sell your science not your hyper-activity
-I previously got a grant. Therefore you should give me another one.
Grants are inputs not outputs.
Tuesday, January 26, 2010
Bridging the chemistry-physics divide
Monday, January 25, 2010
Are flourescent proteins exotic?


Sunday, January 24, 2010
How I use the h-index
Saturday, January 23, 2010
Searching for a unified description of charge transport in molecular materials
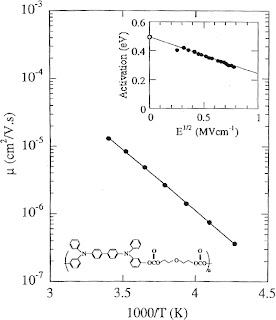

Chemical Reviews just released a special issue on Materials for Electronics. There is a helpful article by David Weiss and Martin Abkowitz, Advances in Organic Photoconductor Technology. They note:
The story of the development of electrophotography is an object lesson in the connections between technology development, product development, and scientific understanding.
Friday, January 22, 2010
Rules for writing any paper
Thursday, January 21, 2010
Mixed valence: physicists vs. chemists II
Wednesday, January 20, 2010
Electronic correlations in cerium oxides
The danger of complaining
Tuesday, January 19, 2010
Provocative quote
Why can undergraduates be taught nuclear physics?

Sunday, January 17, 2010
Mixed valence: physicists vs. chemists I
Saturday, January 16, 2010
A promising career cut short?
Friday, January 15, 2010
Desperately seeking spin liquids II
Wednesday, January 13, 2010
A signature of resonating valence bonds
 The ground state (which has A1g symmetry) can be described as a linear superposition of two valence bond structures, Kr + Kl.
The ground state (which has A1g symmetry) can be described as a linear superposition of two valence bond structures, Kr + Kl.Tuesday, January 12, 2010
You are too polite!
Monday, January 11, 2010
A basic question about novel energy materials

Saturday, January 9, 2010
Correlation does not imply causality
Friday, January 8, 2010
PC vs. Mac
Wednesday, January 6, 2010
A mark of academic freedom?
Monday, January 4, 2010
Are quasi-particles real?
quasi-particles are as real as a share value at the stock exchange. The share value is also due to a collective effect ...,namely to the the collective behavior of all investors. It is also possible to `spray' the share value in Hacking's sense, that is, to manipulate its quotation by purchase or sale for purposes of speculation. Its free fall can make an economy crash, its dramatic rise may make some markets flourish. And the crash as well as the flourishing may be local, i.e., they may only affect some local markets. But would we conclude that the share value does not exist, on the sole grounds that it is a collective effect? Obviously, share values as well as quasi-particles have another ontological status than, say, Pegasus. Pegasus does not exist in the real world but only in the tales of antique mythology. But quasi-particles exist in real crystals, as share values exist in real economies and markets. Indeed, both concepts have a well-defined operational meaning, even though their cause cannot be singled out by experiments or econometric studies.
Friday, January 1, 2010
Evidence for climate change
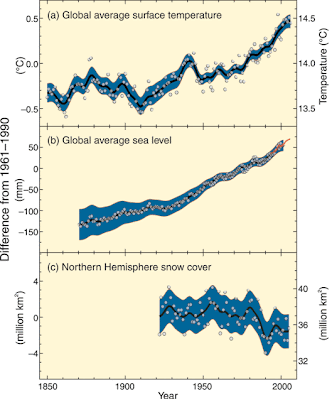
I increasingly get asked by non-scientist friends "Do scientists really believe in climate change?". Consequently, I am trying to be better informed. I found this Summary for Policymakers from the Intergovernmental Panel on Climate Change (IPCC) helpful. The Figure above is taken from that report.
Thermodynamics and emergence
Novelty. Temperature and entropy are emergent properties. Classically, they are defined by the zeroth and second laws of thermodynamics, re...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...
-
I welcome discussion on this point. I don't think it is as sensitive or as important a topic as the author order on papers. With rega...

