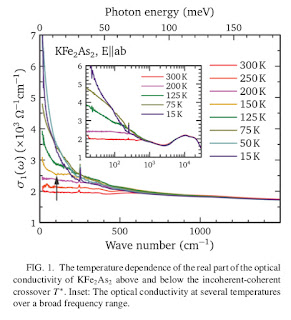
An important question concerning multi-band systems with Hund's rule coupling, such as iron-based superconductors, is whether there is a similar collapse of the Drude peak. This is clearly seen in one material in a recent paper
Observation of an emergent coherent state in the iron-based superconductor KFe2As2
Run Yang, Zhiping Yin, Yilin Wang, Yaomin Dai, Hu Miao, Bing Xu, Xianggang Qiu, and Christopher C. Homes
Note how as the temperature increases from 15 K to 200 K that the Drude peak collapses.
The authors give a detailed analysis of the shifts in spectral weight with varying temperature by fitting the optical conductivity (and reflectivity from which it is derived) at each temperature to a model consisting of three Drude peaks and two Lorentzian peaks. Note this involves twelve parameters and so one should always worry about the elephants trunk wiggling.
On the other hand, they do the fit without the third peak, which is of the greatest interest as it is the sharpest and most temperature dependent, and claim it cannot describe the data.
The authors also perform DFT+DMFT calculations of the one-electron spectral function (but not the optical conductivity) and find it does give a coherent-incoherent crossover consistent with the experiment. However, the variation in quasi-particle weight with temperature is relatively small.