At the end of 2009 I posted the most viewed posts on this blog. Here is the list from 2010. The number of pageviews are from Google Analytics and are an under-estimate.
1. Nature publishes 17 parameter fit to 20 data points 2,900 pageviews
2. There is no perfect Ph.D project 1,000
3. A Ph.D without scholarship? 310
4. Breakdown of the Born-Oppenheimer approximation 300
5. Beware of curve fitting 280
6. OPV cell efficiency is an emergent property 230
7. Artificial photosynthesis 224
8. Want ad: measure for quantum frustration 180
9. 100 most influential living British scientists 176
10. Ph.D without knowledge 154
Wednesday, December 29, 2010
Sunday, December 26, 2010
Science or metaphor?
I have just started reading a beautiful little book by Denis Noble entitled The Music of Life: Biology beyond genes. It highlights the limitations of a reductionistic approach to biology and the value of an emergent perspective, as in systems biology.
Some of the material is in an earlier article. He considers the following two paragraphs which both discuss the role of genes in an organism:
Which do you agree with? Is there any experiment which could be used to distinguish the scientific validity of the two statements?
Some of the material is in an earlier article. He considers the following two paragraphs which both discuss the role of genes in an organism:
Now they swarm in huge colonies, safe inside gigantic lumbering robots, sealed off from the outside world, communicating with it by tortuous indirect routes, manipulating it by remote control. They are in you and me; they created us, body and mind; and their preservation is the ultimate rationale for our existence.
Now they are trapped in huge colonies, locked inside highly intelligent beings, moulded by the outside world, communicating with it by complex processes, through which, blindly, as if by magic, function emerges. They are in you and me; we are the system that allows their code to be read. and their preservation is totally dependent on the joy we experience in reproducing ourselves (our joy not theirs!) We are the ultimate rationale for their existence.
Thursday, December 23, 2010
A veteran teacher shares his wisdom
David Griffiths taught at Reed College for 30 years (a rather unique undergraduate institution in Portland, Oregon) and is author of several widely used textbooks. He has a provocative piece Illuminating physics for students in Physics World. [I first encountered the article on the noticeboard outside the Mott lecture theatre at Bristol University]. The summary is:
He says that the role of a physics teacher should be to illuminate the subject's intrinsic interest, beauty and power – and warns that attempts to make it more marketable using gimmicks, false advertising or dilution are bound to be counterproductiveIt is worth reading in full. But here are a few extracts to picque your interest:
What we have on offer is nothing less than an explanation of how matter behaves on the most fundamental level. It is a story that is magnificent (by good fortune or divine benevolence), coherent (at least that is the goal), plausible (though far from obvious) and true (that is the most remarkable thing about it). It is imperfect and unfinished (of course), but always improving. It is, moreover, amazingly powerful and extraordinarily useful. Our job is to tell this story ....
[clickers] can be powerfully effective in the hands of an inspired expert like Mazur, but I have seen them reduced to distracting gimmicks by less-capable instructors. What concerns me, however, is the unspoken message reliance on such devices may convey: (1) this stuff is boring; and (2) I cannot rely on you to pay attention. Now, point (2) may be valid, but point (1) is so utterly and perniciously false that one should, in my view, avoid anything that is even remotely open to such an interpretation.....
I have never suffered the interference of a brainless dean concerned only with grants and publications, and as a consequence I have been more productive than would have been possible in the usual academic straitjacket. I do not know what makes good teaching, beyond the obvious things: absolute command of the subject; organization; preparation (I write out every lecture verbatim the night before, though I never bring my notes to the lecture hall); clarity; enthusiasm; and a story-teller's instinct for structure, pacing and drama. I personally never use transparencies or PowerPoint – these things are fine for scientific talks, but not in the classroom. ....
Wednesday, December 22, 2010
Tuneable electron-phonon scattering graphene
There is a nice article by Michael S. Fuhrer in Physics about tuning the Fermi surface area in graphene and using it to observe qualitatively different temperature dependence of the resistivity due to electron-phonon scattering.
Tuesday, December 21, 2010
Good internet access while travelling in the USA
In the past this has been an issue. But, last trip at Radio Shack I bought one of these Virgin Broadband2Go devices. The rates have now decreased so that on this trip I am paying just $40 for a month of unlimited access. The coverage is pretty good although it is occasionally slow at remoter locations.
Sunday, December 19, 2010
Seeing what you want to see
There is a good Opinion piece in the November Scientific American, Fudge Factor: a look at Harvard science fraud case by Scott O. Lillienfeld. He discusses the problem of distinguishing intentional scientific fraud from confirmation bias, the tendency we have as scientists to selectively interpret data in order to confirm our own theories.
This is a good reminder that the easiest person to fool is yourself.
This is a good reminder that the easiest person to fool is yourself.
Friday, December 17, 2010
Not everything is RVB
The pyrochlore lattice consists of a three-dimensional network of corner sharing tetrahedra. In a number of transition metal oxides the metal ions are located
on a pyrochlore lattice.
The ground state of the antiferromagnetic Heisenberg model on a pyrochlore lattice is a gapped spin liquid (see this PRB by Canals and Lacroix). The ground state consists of weakly coupled RVB (resonating valence bond) states on each tetrahedra. The conditions necessary for deconfined spinons has been explored in Klein type models on the pyrochlore lattice.
The material KOs2O6 has a pyrochlore structure and was discovered to be superconducting with a transition temperature of about 10 K. Originally it was thought (and hoped) that the superconductivity might be intimately connected to RVB physics. However, it now seems that the superconductivity is not unconventional. It can be explained in terms of strong coupling electron-phonon interaction which arises because of anharmonic phonons associated with ``rattling" vibrational modes of the K ions which are located inside relatively large spatial regions within the cage of Os and O ions (see this paper).
on a pyrochlore lattice.
The ground state of the antiferromagnetic Heisenberg model on a pyrochlore lattice is a gapped spin liquid (see this PRB by Canals and Lacroix). The ground state consists of weakly coupled RVB (resonating valence bond) states on each tetrahedra. The conditions necessary for deconfined spinons has been explored in Klein type models on the pyrochlore lattice.
The material KOs2O6 has a pyrochlore structure and was discovered to be superconducting with a transition temperature of about 10 K. Originally it was thought (and hoped) that the superconductivity might be intimately connected to RVB physics. However, it now seems that the superconductivity is not unconventional. It can be explained in terms of strong coupling electron-phonon interaction which arises because of anharmonic phonons associated with ``rattling" vibrational modes of the K ions which are located inside relatively large spatial regions within the cage of Os and O ions (see this paper).
To me this is a cautionary tale in my enthusiasm for RVB physics.
Thursday, December 16, 2010
The challenge of H-bonding
Hydrogen bonds are ubiquitous in biomolecules and a key to understanding their functionality. This was first appreciated by Pauling and exploited by Watson and Crick to decode the structure of DNA. It is also the origin of the unique and amazing properties of water. There is a helpful review article Hydrogen bonding in the solid state by Steiner. Here are a few things I learnt.
Energies vary by 2 orders of magnitude, 0.2-40 kcal/mol [10 meV to 2 eV]. This spans the energy range from van der Waals to covalent and ionic bonds. The amount of electrostatic, covalent, and dispersion character of the bond varies within this range.
For O-H ... O bonds the shift in frequency of the O-H bond correlates with the distance between the oxygen atoms. The O-H distance is correlated with the O..H distance.
All hydrogen bonds can be considered as incipient hydrogen transfer reactions.
Hydrogen bonds exhibit some unexplained isotope effects. Simple zero-point motion arguments suggest that deuterium substitution should lead to weaker bonds, as is observed in some cases. However, some bonds exhibit a negligible effect and others a negative effect.
Energies vary by 2 orders of magnitude, 0.2-40 kcal/mol [10 meV to 2 eV]. This spans the energy range from van der Waals to covalent and ionic bonds. The amount of electrostatic, covalent, and dispersion character of the bond varies within this range.
For O-H ... O bonds the shift in frequency of the O-H bond correlates with the distance between the oxygen atoms. The O-H distance is correlated with the O..H distance.
All hydrogen bonds can be considered as incipient hydrogen transfer reactions.
Hydrogen bonds exhibit some unexplained isotope effects. Simple zero-point motion arguments suggest that deuterium substitution should lead to weaker bonds, as is observed in some cases. However, some bonds exhibit a negligible effect and others a negative effect.
Wednesday, December 15, 2010
Feynman on path integrals for cheap
The book Quantum mechanics and path integrals by Feynman and Hibbs is a classic that was out of print and an old hardback edition is currently going for $799! The good news is the book has been reprinted by Dover and you can now buy a copy on Amazon for only US$12. My copy arrived today.
Tuesday, December 14, 2010
Basics of inflation
I quite like the new journal Physics from APS because it has nice overview articles which are particularly good for learning something about topics outside ones expertise. There is a good article Can we test inflationary expansion of the early universe?
It explains the basic ideas behind inflation [including the broken symmetry associated with the inflaton field], why it is necessary in standard big bang cosmology, to solve the "horizon" and "flatness" problems, and the hope of actually finding more than circumstantial evidence for inflation.
It explains the basic ideas behind inflation [including the broken symmetry associated with the inflaton field], why it is necessary in standard big bang cosmology, to solve the "horizon" and "flatness" problems, and the hope of actually finding more than circumstantial evidence for inflation.
Sunday, December 12, 2010
Marrying Heitler-London and Pauling
The Linus Pauling archive at Oregon State University has lots of nice resources including original manuscripts, videos, quotes, and photos. Above is a photo of Heitler and London with Pauling's wife.
Broken symmetry is comical
This comic in the Pearls before Swine series appeared in the newspaper today. I thank my family for bringing it to my attention. The previous days cartoon was about Australia and alternative energy.
Saturday, December 11, 2010
Resonant Raman basics
I am trying to get a better understanding of resonant raman scattering as a probe of properties of excited states of organic molecules. The figure below [taken from a JACS paper] shows how in the Green Flourescent Protein one sees an enhanced coupling to vibrations [blue curve in lower panel].
There is a nice site on Resonant Raman Theory set up by Trevor Dines (University of Dundee). It emphasizes that in the Born-Oppenheimer approximation one only sees a significant signal when the excited state involves a displacement of the vibrational co-ordinate.
This raises a question about whether in a completely symmetric molecule one can have a resonant Raman signal due to effects that go beyond Born Oppenheimer.
Friday, December 10, 2010
What did he know and when did he know it?
This was the key question in Watergate scandal.
But, this post is actually about what a Ph.D student should know and when they should know it. Here are a few different answers I have heard:
When the student knows more about the topic than their advisor/supervisor they are ready to submit their thesis.
At the end of your Ph.D you should know more about the topic than anyone else in the world.
The student should know more about the project than their advisor by the time they do their comprehensive exam (a few years into a US Ph.D).
But, this post is actually about what a Ph.D student should know and when they should know it. Here are a few different answers I have heard:
When the student knows more about the topic than their advisor/supervisor they are ready to submit their thesis.
At the end of your Ph.D you should know more about the topic than anyone else in the world.
The student should know more about the project than their advisor by the time they do their comprehensive exam (a few years into a US Ph.D).
Thursday, December 9, 2010
Simple valence bond model for a chemical reaction
Valence bond theory provides an intuitive picture of not just chemical bonding but bond breaking and making. For a reaction ab + c -> ac + b, one can write done the energy of the total system in terms of pairwise exchange J and coulomb integrals Q. This can be used to produce semi-empirical potential energy surfaces and/or diabatic states and coupling between them. This is at the heart of the treatment of coupled electron-proton transfer by Hammes-Schiffer and collaborators, discussed in previous posts. I struggled a bit to find the background of this. It goes back to London-Eyring-Polanyi-Saito (LEPS). A nice summary is the paragraph below taken from a paper by Kim, Truhlar, and Kreevoy. It provides a way to parametrise the Qs and Js in terms of empirical Morse potentials for the constituent molecules. At the transition state the gap to the next excited state is related to a singlet-triplet gap, a point emphasized by Shaik and collaborators.
More background is in material in the old text, Theoretical Chemistry by Glasstone (1944).
Wednesday, December 8, 2010
Coupled electron-proton transfer
In many biochemical processes electron transfer and proton transfer are important and have featured in many of my posts. However, another process that is important and fascinating is coupled electron proton transfer. This is the process discussed in a previous post about the enzyme soybean lipoxygenase. Sometimes this can be viewed as a hydrogen atom transfer, but in some cases the electron and proton start or end at different sites on the donor or acceptor molecule. Describing this process (even for the H-atom transfer case) theoretically has proven to be a challenge which has recently attracted significant attention. A recent review is by Sharon Hammes-Schiffer. Basic questions that arise include:
- What is the reaction co-ordinate? Is it the proton (or H-atom) position? Or the solvent (or heavy atoms) configuration? Or both?
- What is the role of proton tunneling?
- Are the dynamics of the electron and the proton both adiabatic or non-adiabatic?
- Under what conditions is the electron and proton transfer concerted and when is it sequential?
[The figure above is taken from another review]. The figure below shows some possible mechanisms for the coupled proton-electron transfer between tyrosine and tryptophan, which is important in various biochemical processes. It is taken from here.
Monday, December 6, 2010
Tunneling without instantons?
This attempts to answer questions raised in a previous post.
Here is one point of view.
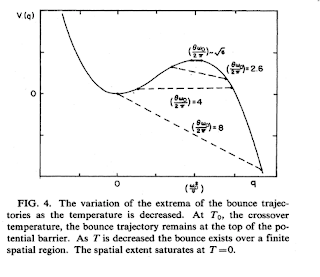
Tunneling is always present and as one lowers the temperature (or increases the coupling to the environment) one just has a crossover from transitions dominated by activation over the barrier to tunneling under the barrier. Instantons [or the "bounce solution" which is a solution to the classical equations of motion in an inverted potential] are just a convenient calculational machinery which arises when evaluating a path integral approximately by finding saddle points. There is always a contribution from the trivial solution corresponding to the top of the barrier. Quadratic fluctuations about this saddle point give a "prefactor" which includes quantum corrections due to tunneling and reflection.
Here is one point of view.
Tunneling is always present and as one lowers the temperature (or increases the coupling to the environment) one just has a crossover from transitions dominated by activation over the barrier to tunneling under the barrier. Instantons [or the "bounce solution" which is a solution to the classical equations of motion in an inverted potential] are just a convenient calculational machinery which arises when evaluating a path integral approximately by finding saddle points. There is always a contribution from the trivial solution corresponding to the top of the barrier. Quadratic fluctuations about this saddle point give a "prefactor" which includes quantum corrections due to tunneling and reflection.
Below the crossover temperature T0 this first saddle point becomes unstable and there is a second saddle point, which is the instanton solution.
I thank Eli Pollak for sharing his thoughts on this subject.
But all this seems against the spirit of the approach to tunneling in dissipative environments, pioneered by Leggett [and reviewed in detail here], which seems to assert that tunneling only exists when instanton solutions are present.
Perhaps, the key distinction is that the instanton captures coherent tunneling whereas the quadratic fluctuations only capture incoherent tunneling. Specifically, if one considers a double well system, the instanton can capture the level splitting associated with tunneling.
Perhaps, the key distinction is that the instanton captures coherent tunneling whereas the quadratic fluctuations only capture incoherent tunneling. Specifically, if one considers a double well system, the instanton can capture the level splitting associated with tunneling.
I am keen to hear others perspectives.
Saturday, December 4, 2010
Deconstructing H atom transfer in enzymes
Yesterday I had a really helpful discussion with Judith Klinman about the question of quantum tunneling of hydrogen in enzymes. [An accessible summary of her point of view is a recent Perspective with Zachary Nagel in Nature Chemical Biology].
Here are a few points I came to a better appreciation of:
There are a number of enzymes (e.g. soybean lipoxygenase) which have very small activation energies (Ea~0-2 kcal/mol ~ 100 meV) for hydrogen atom transfers. (n.b. this is a coupled electron and proton transfer). They exhibit kinetic isotope effects which are
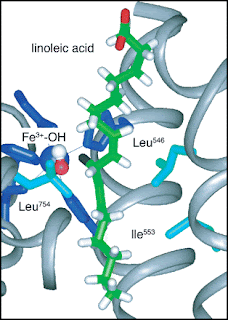
[In the figure above the hydrogen atom (black in the centre of the figure) is transfered to the oxygen atom (red, to the left of the H atom). Mutations correspond to substituting the amino acids Ile553 and/or Leu754.]
The key physics is the following (originally proposed by Kutzenov and Ulstrup) which might be viewed as the proton version on Marcus-Hush electron transfer theory. A JACS paper by Hatcher, Soudakov, and Hammes-Schiffer gives a more sophisticated treatment, including molecular dynamics simulations to extract model parameter values.
The proton directly tunnels between the vibrational ground states of the reactant and product. The isotope effect arises because the spatial extent of the vibrational wavefunction is different for the two isotopes. The temperature dependence of the isotope effect is determined by vibrations of the relative positions of the donor and acceptor atoms.
The main problem or challenge that this model has is the following. The simplest model treatment deduces that the tunneling distance is 0.66 Angstroms [which is less than the van der Waals radii?] and that this increases significantly, up to 2.6 A with mutations. Hammes-Schiffer gets smaller variations, which are more realistic.
However, recent determinations of the crystal structures of the mutants show some structural changes but they do not clearly correlate with the changes in kinetics. In particular there are no detectable changes in tunneling distance. Klinman takes this as evidence for the important role of dynamics.
But, perhaps these structural changes lead to changes in the potential energy surface which in turn changes the amount of tunneling. Things I would like to see include:
-DFT calculations of the potential energy surfaces for the different mutations
-an examination of the Debye-Waller factors for the donor and acceptor atoms in the different mutant structures.
-an examination of non-Born-Oppenheimer effects [which will be isotope dependent].
Note added later: I just found a recent paper by Edwards, Soudakov, and Hammes-Schiffer which uses molecular dynamics to show how the mutations change the tunneling distance and frequency and consequently the kinetic isotope effect. The abstract figure is below.
Here are a few points I came to a better appreciation of:
There are a number of enzymes (e.g. soybean lipoxygenase) which have very small activation energies (Ea~0-2 kcal/mol ~ 100 meV) for hydrogen atom transfers. (n.b. this is a coupled electron and proton transfer). They exhibit kinetic isotope effects which are
- very large in magnitude (~100)
- weakly temperature dependent (difference in Ea for H and D ~ 1 kcal/mol ~ 50 meV)
- change their temperature dependence significantly with mutation
[In the figure above the hydrogen atom (black in the centre of the figure) is transfered to the oxygen atom (red, to the left of the H atom). Mutations correspond to substituting the amino acids Ile553 and/or Leu754.]
The key physics is the following (originally proposed by Kutzenov and Ulstrup) which might be viewed as the proton version on Marcus-Hush electron transfer theory. A JACS paper by Hatcher, Soudakov, and Hammes-Schiffer gives a more sophisticated treatment, including molecular dynamics simulations to extract model parameter values.
The proton directly tunnels between the vibrational ground states of the reactant and product. The isotope effect arises because the spatial extent of the vibrational wavefunction is different for the two isotopes. The temperature dependence of the isotope effect is determined by vibrations of the relative positions of the donor and acceptor atoms.
The main problem or challenge that this model has is the following. The simplest model treatment deduces that the tunneling distance is 0.66 Angstroms [which is less than the van der Waals radii?] and that this increases significantly, up to 2.6 A with mutations. Hammes-Schiffer gets smaller variations, which are more realistic.
However, recent determinations of the crystal structures of the mutants show some structural changes but they do not clearly correlate with the changes in kinetics. In particular there are no detectable changes in tunneling distance. Klinman takes this as evidence for the important role of dynamics.
But, perhaps these structural changes lead to changes in the potential energy surface which in turn changes the amount of tunneling. Things I would like to see include:
-DFT calculations of the potential energy surfaces for the different mutations
-an examination of the Debye-Waller factors for the donor and acceptor atoms in the different mutant structures.
-an examination of non-Born-Oppenheimer effects [which will be isotope dependent].
Note added later: I just found a recent paper by Edwards, Soudakov, and Hammes-Schiffer which uses molecular dynamics to show how the mutations change the tunneling distance and frequency and consequently the kinetic isotope effect. The abstract figure is below.
Friday, December 3, 2010
Kagome lattice antiferromagnet may be a spin liquid
As I have posted previously finding a realistic Heisenberg spin model Hamiltonian which has a spin liquid ground state has proven to be difficult. The model on the Kagome lattice was thought to be a prime candidate for many years, partly because the classical model has an infinite number of degenerate ground states. However, a few years ago Rajiv Singh and David Huse performed a series expansion study which suggested that the ground state was actually a valence bond crystal with a unit cell of 36 spins. In the picture below the blue lines represent spin singlets, and H, P, and E, denote Hexagons, Pinwheels, and Empty triangles respectively. This result was confirmed by my UQ colleagues Glen Evenbly and Guifre Vidal using a completely different numerical method based on entanglement renormalisation.
However, there are new numerical results using DMRG which appeared on the arXiv this week, by Yan, Huse, and White. They find a spin liquid ground state, with a gap to both singlet and triplet excitations.
Interlayer magnetoresistance as a probe of Fermi surface anisotropies
Here are the slides for a talk I will give in the physics department at Berkeley this afternoon. Some of what I will talk about concerns a paper with Michael Smith, which appeared online in PRB this week, Fermi surface of underdoped cuprate superconductors from interlayer magnetoresistance: Closed pockets versus open arcs
Thursday, December 2, 2010
Ambiguities about tunneling at non-zero temperature?
An important question came up in the seminar I gave today. It concerns something I have been confused about for quite a while and I was encouraged that some of the audience got animated about it.
Consider the problem of quantum tunneling out of the potential minimum on the left in the Figure below
However, there is an alternative way to obtain the same expression, which seems to me to involve assuming lots of tunneling! Eli Pollak [who is also visiting Berkeley] reminded me of this today. I think this derivation was originally due to Bell, following earlier work by Wigner, and is implicit in Truhlar and collaborators treatment of tunneling and implemented in their POLYRATE code. One considers the tunneling transmission amplitude as a function of energy and integrates over all energies with a Boltzmann weighting factor.
How does one reconcile these two derivations, which seem to me to involve very different physics? I welcome comments.
Consider the problem of quantum tunneling out of the potential minimum on the left in the Figure below
If one considers a path integral approach than one says that tunneling occurs when there is an "instanton" solution (i.e., a non-trivial solution) to the classical equations of motion in imaginary time with a period determined by the temperature. This only occurs when the temperature is less than
which is defined by curvature of the top of the potential barrier. At temperatures above this there is only one solution to the classical equations of motion, the trivial one x(tau)=x_b, corresponding to the minima of the inverted potential. One can then calculate the quantum fluctuations about this minimum, at the Gaussian level. This gives a total decay rate
which is well defined, provided the temperature T is larger than T_0. What do these quantum fluctuations represent? I would say they represent tunneling just below the top of the barrier and reflection from just above the top of the barrier. In my talk I said this situation of "no instantons" represents "no tunneling" which several people disagreed with. I corrected myself with "no deep tunneling."However, there is an alternative way to obtain the same expression, which seems to me to involve assuming lots of tunneling! Eli Pollak [who is also visiting Berkeley] reminded me of this today. I think this derivation was originally due to Bell, following earlier work by Wigner, and is implicit in Truhlar and collaborators treatment of tunneling and implemented in their POLYRATE code. One considers the tunneling transmission amplitude as a function of energy and integrates over all energies with a Boltzmann weighting factor.
How does one reconcile these two derivations, which seem to me to involve very different physics? I welcome comments.
Revival of the non-Fermi liquid
On the Condensed Matter Physics Journal Club Patrick Lee has a nice summary of recent work concerning very subtle problems concerning large N expansions of gauge theories with non-Fermi liquid ground state.
Chemistry seminar at Berkeley
I am giving a seminar, Limited role of quantum dynamics in biomolecular function in the Chemistry department at Berkeley this afternoon.
Wednesday, December 1, 2010
An ugly period in American physics/politics
On the plane from Brisbane to LA I watched some of The Trials of J. Robert Oppenheimer [you can also watch it online] which gave an excellent portrayal (using exact testimony) of the trial and background that led to Oppenheimer losing his security clearance. It also had some excellent background on Oppenheimer's youth and time as a young faculty member at Berkeley. [Mental health issues feature somewhat].
Other physicists who were "tarred and feathered" in the McCarthy era were David Bohm [who was basically fired by Princeton University because he refused to testify] and Frank Oppenheimer [younger brother of J. Robert] who was forced to resign from a faculty position at University of Minnesota. He later founded the Exploratorium in San Francisco.
Last year Physics Today published a fascinating article by J.D. Jackson [of electrodynamics textbook fame], Panosky agonistes: The 1950 loyalty oath at Berkeley, which chronicles more problems from that era.
[Coincidentally, this post is being written in Berkeley!].
Another aside: the photo at the top is of the audience for a colloquium at Los Alamos during the Manhattan project. See who you can recognise.
Other physicists who were "tarred and feathered" in the McCarthy era were David Bohm [who was basically fired by Princeton University because he refused to testify] and Frank Oppenheimer [younger brother of J. Robert] who was forced to resign from a faculty position at University of Minnesota. He later founded the Exploratorium in San Francisco.
Last year Physics Today published a fascinating article by J.D. Jackson [of electrodynamics textbook fame], Panosky agonistes: The 1950 loyalty oath at Berkeley, which chronicles more problems from that era.
[Coincidentally, this post is being written in Berkeley!].
Another aside: the photo at the top is of the audience for a colloquium at Los Alamos during the Manhattan project. See who you can recognise.
Modelling electron transfer in photosynthesis
This follows up on a previous post about measurements of the rate at which electron transfer occurs in a photosynthetic protein. I noted several deviations of the experimental results from what is predicted by Marcus-Hush electron transfer theory. This is not necessarily surprising because one is not in quite in the right parameter regime.
In principle [at least to me] this should be described by a spin boson model which has the Hamiltonian
and the spectral density contains all the relevant information about the protein dynamics,
So the question I have is: if one has the correct parameters and spectral density can one actually describe all the experiments? Below is the spectral density found by Parson and Warshel in molecular dynamics simulations.
Subscribe to:
Posts (Atom)
Thermodynamics and emergence
Novelty. Temperature and entropy are emergent properties. Classically, they are defined by the zeroth and second laws of thermodynamics, re...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
I welcome discussion on this point. I don't think it is as sensitive or as important a topic as the author order on papers. With rega...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...