Saturday, July 31, 2010
Floppy organic LEDs
A sleepless seminar in Seattle
Thursday, July 29, 2010
Jumping from one surface to another
- Ehrenfest mean-field dynamics
- Surface hopping
- Ehrenfest + decoherence (Prezhdo, Rossky, Schwartz...)
- Semi-classical dynamics (Miller–Meyer–Stock–Thoss formalism)
- Partial Wigner transforms (Kapral)
- Multiple spawning
- Full nuclear quantum dynamics
Wednesday, July 28, 2010
The next Einsteins?

Monday, July 26, 2010
A century after van der Waals Nobel Prize
Sunday, July 25, 2010
AC or DC?
Saturday, July 24, 2010
Lectures on chemical dynamics
 At the 2009 Telluride School on Theoretical Chemistry John Tully (Yale) gave a series of lectures on Chemical Dynamics (both quantum and classical). The slides look very clear and helpful.
At the 2009 Telluride School on Theoretical Chemistry John Tully (Yale) gave a series of lectures on Chemical Dynamics (both quantum and classical). The slides look very clear and helpful.
Solvent-chromophore interactions
Assumption*:for the ground to excited state transition in the chromophore the transition dipole moment is much smaller than the difference of the dipole moments between the ground and excited states.
- the absorption and emission lines obey the mirror image rule
- in the "high" temperature limit (which is reasonable for most solvents at room temperature) the line width is proportional to the Stokes shift and to the temperature
- the reorganisation energy is much less than the transition energy[this just means one can clearly resolve the absorption and emission bands]
- the solvent degrees of freedom are treated classically.
Friday, July 23, 2010
Best paper title and abstract ever?
On Unjustifiably Misrepresenting the EVB Approach While Simultaneously Adopting It
In recent years, the EVB has become a widely used tool in the QM/MM modeling of reactions in condensed phases and in biological systems, with ever increasing popularity. However, despite the fact that its power and validity have been repeatedly established since 1980, a recent work (Valero, R.; et al. J. Chem. Theory Comput. 2009, 5, 1) has strongly criticized this approach, while not discussing the fact that one of the authors is effectively using it himself for both gas-phase and solution studies. Here, we have responded to the most serious unjustified assertions of that paper, covering both the more problematic aspects of that work and the more complex scientific aspects. Additionally, we have demonstrated that the poor EVB results shown in Valero et al. which where presented as verification of the unreliability of the EVB model were in fact obtained by the use of incorrect parameters, without comparing to the correct surface obtained by our program.
Big questions in science
Is it possible to create magnetic semiconductors that work at room temperature?
What is the pairing mechanism behind high-temperature superconductivity?
Can we develop a general theory of the dynamics of turbulent flows and the motion of granular materials?
Is superfluidity possible in a solid? If so, how?
What is the structure of water?
What is the nature of the glassy state?
Are there limits to rational chemical synthesis?
What is the ultimate efficiency of photovoltaic cells?
Can we predict how proteins will fold?
Please scan the lists and let me know what should and should not be there.
Thursday, July 22, 2010
Limited role of quantum dynamics in biomolecular function
Wednesday, July 21, 2010
Deconstructing claims about multiple carrier generation in quantum dots
uncontrolled photocharging of the nanocrystal core can lead to exaggeration of the Auger decay component and, as a result, significant deviations of the apparent [carrier multiplication] CM efficiencies from their true values. Specifically, we observe that for the same sample, apparent multiexciton yields can differ by a factor of3 depending on whether the nanocrystal solution is static or stirred.
Tuesday, July 20, 2010
Important things you learn at workshops

The challenge of water

water reorientation mechanism involves large-amplitude angular jumps, rather than the commonly accepted sequence of small diffusive steps, and therefore calls for reinterpretation of many experimental data wherein water rotational relaxation is assumed to be diffusive.
Monday, July 19, 2010
Condensed phase quantum dynamics
Saturday, July 17, 2010
No-collapse quantum theory
(i) the universal validity of unitary dynamics and the superposition principle has been confirmed far into the mesoscopic and macroscopic realm in all experiments conducted thus far;
(ii) all observed ‘‘restrictions’’ can be correctly and comple
tely accounted for by taking into account environmental decoherence effects;
(iii) no positive experimental evidence exists for physical state-vector collapse

"Ohhhhhhh. ... Look at that, Schuster. ... Dogs are so cute when they try to comprehend quantum mechanics."
What are the assumptions in this minimal theory?
First, a completely known (pure) state of an isolated quantum system is described by a normalized state vector in a Hilbert space.
Second, the time evolution of a state vector is given by the time-dependent Schrodinger equation.
No mention is made of measurements in this formulation. Instead, measurements are described without special axioms in terms of physical interactions between systems described by state vectors (wave functions) and governed by suitable interaction Hamiltonians. Observables then emerge as a derived concept.
Basically, I agree with these and it is nice to see the arguments set out so clearly. On the other hand, I don't follow some of Max's arguments about the amount of entanglement in the macroscopic states that he discusses.
Friday, July 16, 2010
A poor metric of research quality
Hawking's cat had a Nazi genealogy
Thursday, July 15, 2010
Justifying a common mantra
A useful file sharing resource for collaborations
Wednesday, July 14, 2010
The limited relevance of quantum coherence in photosynthesis
Tuesday, July 13, 2010
Perfectionism and procrastination
The Dimensions of perfectionism
Frost et al., Cognitive Theory and Research 14, 449 (1990) [cited 500+ times]
• Excessive concern about making mistakes
• High personal standards
• Perception of high parental expectations
• Perception of high parental criticism
• Doubting of the quality of ones actions
• Preference for order and organisation
An interesting study [which may have never been cited!] I found in ISI but not online claims "Surprisingly, graduate students may procrastinate on academic tasks even more than do undergraduate students. Perfectionism also has been found to be high among graduate students."
Monday, July 12, 2010
A truly quantum phase transition
 Many quantum critical points in d spatial dimensions are expected to have properties similar to corresponding classical critical points in d+1 dimensions.
Many quantum critical points in d spatial dimensions are expected to have properties similar to corresponding classical critical points in d+1 dimensions.Saturday, July 10, 2010
Frauenfelder rules for an effective workshop
Friday, July 9, 2010
Computational photochemistry tutorial

A very effective way to start learning what computational chemistry can and cannot do is to start doing hands on tutorials of concrete examples. Last week, at the I2CAM workshop on photovoltaics, Jeff Reimers ran a hands on workshop where participants calculated photochemical properties associated with charge separation and recombination in a specific donor-acceptor molecule. Here is the tutorial sheet and you can run Jeff's CNDO (Complete Neglect of Differential Overlap) [something like a Hubbard model..] code on the nanohub.
Make your own Vuvuzela sound
Thursday, July 8, 2010
Disentangling extraordinary claims about photosynthesis
The fact that exciton states may be delocalized among chromophores is obviously interesting and implies quantum communication between them, provided it survives decoherence effects due to the surrounding medium. One could then justifiably argue that the chromophores are entangled and that the dynamics is profoundly quantum in nature. However, as shown here, these quantum effects can be easily eliminated by basing the description on the delocalized exciton modes.
Opportunities for theorists
Molecular Understanding of Organic Solar Cells: The Challenges
by Bredas, Norton, Cornil, and Coropceanu.
 It reviews our limited understanding of five key processes in an organic solar cell
It reviews our limited understanding of five key processes in an organic solar cellAre you a wimp?
"Only wimps study the general case. Real scientists work through examples."
Beresford Parlett
This is often quoted by Sir Michael Berry FRS. Although it does not rate a mention on his interesting quotations page.
I thank Ben Powell for bringing the quote to my attention.
Wednesday, July 7, 2010
Deconstructing thermal transport in the pseudogap state
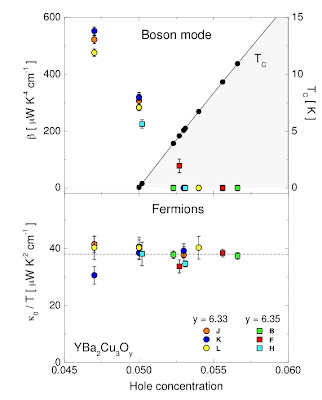 This fact that the thermal conductivity is a powerful probe of the pseudogap state led Michael Smith and I to examine how different models for the pseudogap state (particularly arcs vs. pockets) lead to qualitatively different predictions for the thermal conductivity. Our results appeared in Phys. Rev. B this week.
This fact that the thermal conductivity is a powerful probe of the pseudogap state led Michael Smith and I to examine how different models for the pseudogap state (particularly arcs vs. pockets) lead to qualitatively different predictions for the thermal conductivity. Our results appeared in Phys. Rev. B this week.
Boulder school lectures online
This year the School will again employ a webcast system that allows for real-time streaming of video/audio of its proceedings, including all the lectures, questions, answers, and discussions.
Tuesday, July 6, 2010
Who should I get to write a letter of reference?
Monday, July 5, 2010
Virtual drug design?

To what extent does computational chemistry actually guide drug design?
This came up in the previous post concerning "virtual screening" of candidate molecules for organic photovoltaics.
Seth Olsen brought to my attention a nice article, Virtual Screening: an endless staircase, by Gisbert Schneider that appeared earlier this year in Nature Reviews Drug Discovery. Here is part of the abstract:
Computational chemistry — in particular, virtual screening — can provide valuable contributions in hit- and lead-compound discovery. Numerous software tools have been developed for this purpose. However, despite the applicability of virtual screening technology being well established, it seems that there are relatively few examples of drug discovery projects in which virtual screening has been the key contributor.
Furthermore, the article says:
Substantial progress in virtual screening requires a profound understanding of the forces that govern protein folding and the dynamics of macromolecular complex formation.
The figure above is taken from a this paper about the action of aspirin.
Sunday, July 4, 2010
OPV cell efficiency is an emergent property
Saturday, July 3, 2010
Splitting singlets to save the planet

Friday, July 2, 2010
Understanding properties of dye-sensitizers

At the conference today, my colleague Seth Olsen gave a talk Stucture-Property Relationships for Conjugated Organic Dyes.
Deconstructing excited state dynamics in conjugated polymers
Thursday, July 1, 2010
Charge transport in organic photovoltaic materials
- What is the mechanism of charge transport?
- What is the origin of the observed electric field dependent mobility?
- What determines the relative magnitude of electron and hole mobilities?
- How does mobility depend on the intermolecular separation and relative orientation?
- Would thermopower measurements be helpful in determining the charge transport mechanism?
What Americans might want to know about getting a job in an Australian university
Universities and scientific research in the USA are facing a dire future. Understandably, some scientists are considering leaving the USA. I...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...
-
I welcome discussion on this point. I don't think it is as sensitive or as important a topic as the author order on papers. With rega...


