I wish to highlight some common issues that occur in the construction, justification and parametrisation of effective Hamiltonians in both theoretical chemistry and solid state physics.
The basic issue is one needs to keep in mind that just because one gets the energy eigenvalues of a quantum system "correct" does not mean that one necessarily has the correct wave function.
Previously, I
posted how sometimes a variational wave function can give a good ground state energy but be qualitatively incorrect.
For molecular systems a powerful approach to understanding the potential energy surfaces of the ground state and the lowest lying electronic states is to construct a Hamiltonian matrix based on a few diabatic states.
For crystals in which the electronic degrees of freedom are strongly correlated a powerful approach is to construct a Hubbard model where the non-interacting band structure is described by a tight-binding model. The latter describes hopping of electrons between orbitals that are localised on individual lattice sites.
Quantum chemistry
There are two strategies that are used to construct and parametrise a diabatic state model.
1. Based on chemical insight one writes down a Hamiltonian of the form
One assumes some functional form for the Hamiltonian matrix elements, with several free parameters.
One calculates the adiabatic potential energy surfaces using an ab initio method and fits these surfaces to the adiabatic energies from the diabatic model.
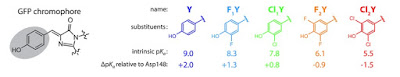
An example is shown below for
a two-state diabatic model for fluorescent protein chromophores.
The example below concerns five electronic states of XH3 and the associated torsional potential, taken from
this paper. There are 11 free parameters in the Hamiltonian.
A different approach by Nangia and Truhlar considers a multi-dimensional potential surface for ammonia, two diabatic states, and hundreds of free parameters.
Important questions arise.
Are the diabatic states physical?
Or is all this curve fitting just
making the elephants trunk to wiggle?
As one includes more diabatic states how does one deal with the confusion and ambiguity that arises because of the close proximity to one another of many excited states?
2. A more rigorous approach is to use some well-defined procedure to actually construct the diabatic states from a knowledge of the many-body wave functions of the low lying states. An example is the
approach pioneered by Cederbaum. Seth Olsen has nicely used this approach to construct diabatic states for
fluorescent protein chromophores and
other organic dyes. Furthermore, the diabatic states can be related to chemically intuitive valence bond structures.
However, subtle issues still arise, particularly as one includes more excited states.
Solid state physics
Similarly, there are two strategies that are used to construct and parametrise
a tight-binding model for a specific material.
1. One writes down a tight-binding model Hamiltonian with a few parameters describing hopping integrals and calculates the associated band structure. One then calculates the band structure for a specific material, using an
ab initio method, usually some approximation of Density Functional Theory (DFT). One then fits this band structure to the tight-binding model in order to determine the hopping integrals.
An example is shown below, taken from this
paper. The green dots are from an DFT based calculation and the solid black lines are a fit to a tight-binding model with a few free parameters.
This procedure gets messy and ambiguous when in order to improve the quality of the fit one starts to introduce extra parameters representing beyond next-nearest neighbour hopping.
Are such long range hoppings justified? Furthermore, the parameter values obtained can vary significantly as one introduces extra parameters.
2. A more rigorous approach is to construct Wannier orbitals and then calculate the actual overlap integrals that are input into a tight-binding model.
An example is in this
paper concerning the Fabre salts. In particular it shows how some longer range hoppings are actually justified.
However, there are many subtleties and ambiguities in this approach as discussed in a recent
Reviews of Modern Physics. This tends to work well when there are a couple of well isolated bands, but not otherwise.
Clearly, 2. is always preferable because it has a stronger physical basis. However, it is not easy. People tend to be just do 1. with a fixed number of parameters and not worry about whether they are justified or stable.
I thank
Seth Olsen and
Anthony Jacko for teaching me about these issues.