- How can something become more "ordered" as the temperature increases?
- Are one-dimensional Ising-type models good for anything?
- How can you distinguish different models for a complex system?
- When and how do biomolecules exhibit collective effects?
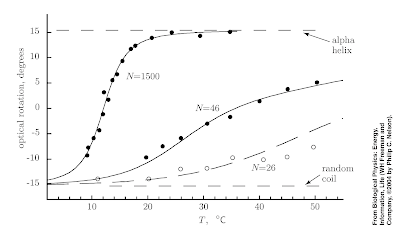
In Chapter 9 of Nelson's Biological Physics answers to these questions are illustrated by the Figure below which shows the temperature temperature of the fraction of a polypeptide chain that form an alpha-helix.
1. The fraction of the chain which forms alpha helices increases with increasing temperature. This is somewhat counter-intuitive. The reason it occurs is although the entropy of the chain decreases with increasing alpha helix content the entropy of the solvent decreases. Formation of alpha helices makes the solvent less ordered because formation of intra-chain hydrogen bonds mean there is less H-bonding of the solvent to the chain.
2. Yes. I remember solving the one-dimensional Ising model in a field when learning "advanced" statistical mechanics at the beginning of my Ph.D. This was considered a warmup for the two-dimensional model and to introduce the transfer matrix technique. However, it turns out the model also describes a one-dimensional polymer chain with an energy cost gamma to backfolding. The "magnetisation" versus temperature curve corresponds to the solid curve in the figure above.
3. Nelson points out that the data for N=1500 is adequately described by the "Ising model" with a large range of gamma values. Hence, the fact that one can fit the models predictions to the data hardly proves the model is correct. But then he goes on to show that the dependence on the chain length is quite sensitive to the gamma value. Indeed the data for all three N values can be described by a model with five parameters. Furthermore, a non-zero value of gamma is crucial showing the importance of interactions and co-operative effects.
4. The fact that the sharpness of the random coil-helix coil transition is sharp for large N is a signature of co-operativity (collective behaviour). Smaller polypeptide chains do not exhibit such a sharp transition. Why does this matter for biomolecular functionality? Because functionality involves turning on and off properties. Hence, biomolecules can undergo significant conformational change in response to a small change in ion concentration, temperature, or local environment.




No comments:
Post a Comment