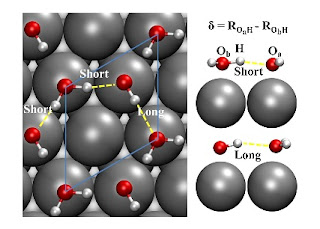
Water is everywhere, even in the air. Consequently, many surfaces (metallic, oxide, and semiconducting) are covered in thin layers of water. It turns out that the first contact layer is actually not pure water, but a mixture of water and hydroxyl (OH-) ions. Furthermore, on a metal surface the separation of the oxygen atoms is largely determined by the lattice constant of substrate [presumably because the oxygen atom lone pairs have a relatively strong interaction with the metal atoms].
On some metals the oxygen atoms are close enough (as in ice under high pressures) that the hydrogen bond between water and hydroxyl ion takes on a covalent character and there is significant delocalisation of the shared proton between the two oxygen atoms.
The above is based on a nice PRL Quantum Nature of the Proton in Water-Hydroxyl Overlayers on Metal Surfaces by Xin-Zheng Li, Matthew I. J. Probert, Ali Alavi, and Angelos Michaelides.
Subscribe to:
Post Comments (Atom)
A forgotten physicist: Amelia Frank (1906-1937)
In honour of International Women's Day, I bring to your attention a fascinating recent piece in The Conversation , Who was Amelia Frank?...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



No comments:
Post a Comment