Previously I posted about how significant redshifts in the frequency of the O-H bond stretch can be a signature of hydrogen bonding. Furthermore, there is a fairly universal relationship between the frequency shift and the bond length and energy.
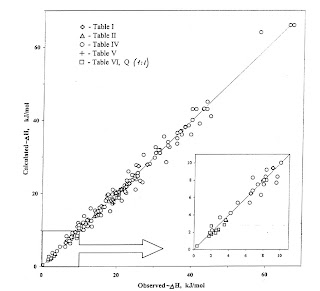
The graph above shows there is also a fairly universal relationship between the bond energy (horizontal scale) and the change in intensity of the O-H infrared absorption intensity, covering a factor of 200 fold variation in the energy. The details are summarised in this paper. The vertical scale is the bond energy calculated from the empirical relation −ΔH=12.2 ΔA1/2 where ΔH is the enthalpy in kJ/mol and ΔA is the change in intensity of the absorption line in units of 10−4 cm mmol−1.
Subscribe to:
Post Comments (Atom)
A forgotten physicist: Amelia Frank (1906-1937)
In honour of International Women's Day, I bring to your attention a fascinating recent piece in The Conversation , Who was Amelia Frank?...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



No comments:
Post a Comment