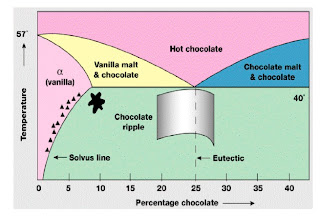
Tomorrow for PHYS2020 Thermodynamics and Condensed Matter Physics I will give a lecture on Phase diagrams.
Hopefully, students will learn that, contrary to what they were taught in high school, there are many phases of matter, not just solid, liquid, and gas!
Furthermore, they begin to see how the Gibbs free energy is the key quantity which defines the relative stability of different phases (at fixed temperature and pressure).
The video demonstrations I use are from the Video Encyclopedia of Physics Demonstrations.
Subscribe to:
Post Comments (Atom)
Information theoretic measures for emergence and causality
The relationship between emergence and causation is contentious, with a long history. Most discussions are qualitative. Presented with a new...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



excellent!
ReplyDelete