Although hydrogen bonded systems (D-H....A) are chemically diverse and have a wide range of physical properties one observes simple empirical correlations between physical properties and two key variables: the donor-acceptor (D-A) distance R and the difference in proton affinity between the donor and acceptor. That is the main starting point of my paper on the subject.
I am continually learning of more empirical correlations that need to be explained.
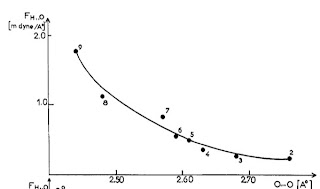
The vibrational frequency for motion of the donor D-H relative to the acceptor A can be measured by infra-red spectroscopy. No particular correlations are observed with R. However, this is because of a diversity of donor and acceptor masses. If instead one uses the frequency and masses to calculate the force constant [the curvature of the hydrogen bond potential] one observes a correlation.
The Figure below is taken from a 1974 review article by Novak.
I believe this correlation is a key ingredient to understanding the secondary geometric isotope effect in hydrogen bonded systems. This is where the donor-acceptor distance changes with deuterium substitution. This is also known as the Ubbelohde effect, after one of the people who discovered it [or at least first highlighted it in 1955]. A portrait of Leo Ubbelohde is shown below. Clearly, he was not very excited about hydrogen bonding!
I thank Angelos Michaelides for bringing the portrait to my attention. When Angelos showed the portrait at the Telluride meeting it caused some amusement.
Correction (15 July, 2014). It turns out this is the wrong Ubbelohde. The correct one is actually Alfred Ubbelohde.





No comments:
Post a Comment