Owing to its pervasive dependence on the superposition phenomenon, chemistry truly deserves to be called “the quantum science”.This is the concluding sentence of a beautiful article Chemical Bonding as a Superposition Phenomenon by Frank Weinhold in the Journal of Chemical Education.
It is a nice easy introduction to physicists who want to start to get some sense of what chemical bonding is all about.
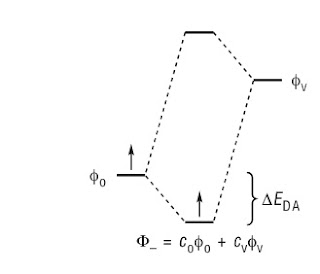
A key idea is that because of the superposition principle that as two chemical units [whether atoms or molecules] are brought closer together, the composite system can lower its energy by being in a superposition state, as illustrated below.
Although the article discusses this idea at the one electron level it is important to realise that this superposition is also relevant for many-body wavefunctions.
The paper also mentions that superposition is at the heart of quantum entanglement, and thus at the heart of quantum weirdness. However, no hints are given of how entanglement may actually play a role in quantum chemistry. That turns out to be a slippery subject, e.g. giving a clear and well-defined measure of entanglement in a simple molecule.
Weinhold's ideas are expanded in great detail in the beautiful book Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective, co-authored with Clark Landis.




No comments:
Post a Comment