I have been trying to learn some of the basics of infra-red spectroscopy of organic compounds and found this site for an organic chemistry lab course at University of Missouri helpful. Why should a quantum many-body theorist care?
Well, it turns out that the frequency, intensity, and lineshape associated with particular chemical bonds are quite sensitive to the type of bonding involved and the local environment of the bond, including valence states, orbital hybridisation, charge distribution, and the presence of resonating valence bond structures.
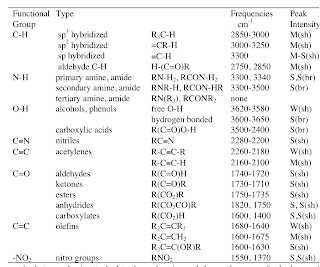
Previously I posted about the vibrational frequencies of O-H stretches associated with hydrogen bonding. The Table below, taken from here illustrates and summarises some of these effects.
But maybe someone could recommend a good book or review article. I struggled to find one online...
Table 1. A summary of the principle infrared bands and their assignments.
R is an aliphatic group.
Well, it turns out that the frequency, intensity, and lineshape associated with particular chemical bonds are quite sensitive to the type of bonding involved and the local environment of the bond, including valence states, orbital hybridisation, charge distribution, and the presence of resonating valence bond structures.
Previously I posted about the vibrational frequencies of O-H stretches associated with hydrogen bonding. The Table below, taken from here illustrates and summarises some of these effects.
But maybe someone could recommend a good book or review article. I struggled to find one online...
Table 1. A summary of the principle infrared bands and their assignments.
R is an aliphatic group.




Hi Ross,
ReplyDeleteThe two books I like on this are "Molecular Spectroscopy" by Flygare and
"Spectrometric identification of organic compounds"--I forget the author.
Flygare's book definitely a theory book written by a spectroscopist and goes in to a lot detail on line shape broadening, etc.
Cheers,
Eric