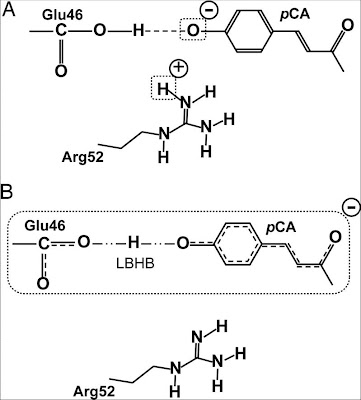
When visiting Irene Burghardt she told me about an important development in our understanding of the chromophore in the Photoactive Yellow Protein (PYP). A recent neutron scattering study [which I wrote a previous blog post about from a different angle] showed that the standard picture (A above) of the charge distribution around the chromophore was incorrect.
It was previously assumed/claimed that the Arg52 and Glu42 amino acids in the protein were protonated and the chromophore was negatively charged. However, the neutron scattering experiment showed that the structures were actually those shown in B above. In particular there is a low barrier hydrogen bond (LBHB) between the Glu42 and the chromophore.
This was also confirmed in an independent NMR study, also recently published in PNAS.




Wow, this is news. Ionization of an Arg residue in this case would be QUITE unusual at physiological pH. The chromophore pocket in PYP is not far from the solution, unlike in GFP, because the chromophore is an externally obtained cofactor.
ReplyDelete