Last year, I gave a talk on this topic at the thursday COPE science meeting. I discussed how it is possible to quantify these spectral shifts in terms of the static dielectric constant of the solvent. It was based on a nice PRL, Solid State Solvation in Amorphous Organic Thin Films.
Such a quantitative analysis should be the starting point for any claims about the origin and cause of solvatochromatic shifts in complex molecular materials.
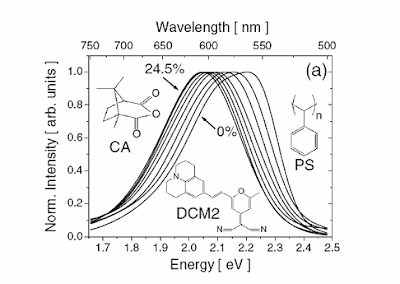




I feel like I've seen this picture before?
ReplyDelete