A while back I asked about How good is the Morse potential at describing the potential energy of a chemical bond as a function of stretching of the bond length?
Today I learnt (from Atkins Physical Chemistry text) that Birge-Sponer extrapolation plots provide a means to test the potential all the way up to the disassociation limit. One plots the difference in energy/frequency between neighbouring transitions. If the plot is linear and then where it extrapolates provides a means to determine the disassociation energy.
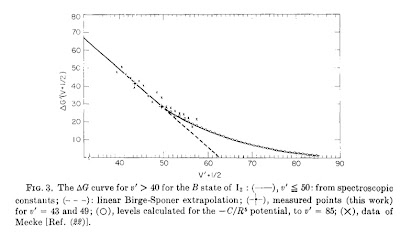
The Figure above shows a plot for an excited state of the iodine diatomic molecule. The plot is linear all the way up to quantum numbers of order 50. It is taken from this paper.
There is a nice article in Journal of Chemical Education which describes how all of this is (or can be) done in undergraduate Physical Chemistry labs. Pity I missed out...
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



No comments:
Post a Comment