I found a very nice paper in Nature Chemistry
Taking Ockham's razor to enzyme dynamics and catalysis
by David R. Glowacki, Jeremy N. Harvey, and Adrian J. Mulholland
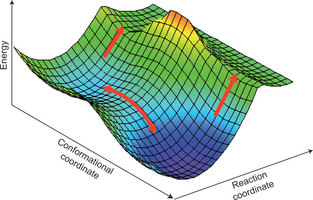
They consider a simple transition state model for the anomalous kinetic isotope effects that have been observed in several enzymes. These anomalies have previously been claimed to be evidence for quantum tunneling, breakdown of transition state theory, and require a new paradigm for enzyme catalysis.
The key feature of their model is the assumption that there are two transition states, not one, being associated with two possible conformations of the enzyme-substrate complex.
They end the paper quoting a 1991 Nature paper by Jeremy Knowles
Enzyme catalysis: Not different, just better.
Another nice reference removing the almost mystical interpretation of proteins [and containing some nice thermodynamics] is
Protein heat capacity: An anomaly that maybe never was by Alan Cooper.
I need to read it.
There is an important lesson here, particularly for proponents of "quantum biology". Extra-ordinary explanations require first ruling out simpler less glamorous explanations.




No comments:
Post a Comment