However, over the past 15 years it has been discovered that there are a class of (very weak) bonds, best described as improper H bonds which are distinctly different. Generally, the donor X is not particularly electronegative (e.g. a carbon atom) and bond formation results in
- contraction of the X-H bond (by a few milliAngstroms)
- hardening of the X-H stretch frequency (by less than one per cent)
- decrease in the X-H stretch IR intensity
There is a nice 2007 JACS paper Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation by Jorly Joseph and Eluvathingal D. Jemmis.
The key idea is as follows
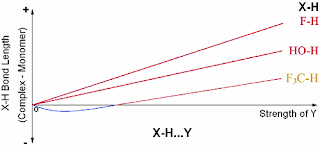
The factors which affect the X−H bond in all X−H···Y HBs can be divided into two parts: (a) The electron affinity of X causes a net gain of electron density at the X−H bond region in the presence of Y and encourages an X−H bond contraction. (b) The well understood attractive interaction between the positive H and electron rich Y forces an X−H bond elongation. For electron rich, highly polar X−H bonds (proper HB donors) the latter almost always dominates and results in X−H bond elongation, whereas for less polar, electron poor X−H bonds (pro-improper HB donors) the effect of the former is noticeable if Y is not a very strong HB acceptor.
In different words due to interaction with the acceptor the relative amount of covalent and ionic character of the X-H bond changes.
Hence, I think this goes beyond my simple two diabatic state picture which does not allow the X-H diabatic state to vary in character with the interaction.
I thank Pranav Shirhatti for stimulating my interest in this problem.




No comments:
Post a Comment