Sharon Hammes-Schiffer gave an interesting talk in Telluride last week about coupled electron-proton transfer.
[A couple of my earlier posts on this fascinating subject are here and here].
Here are a few things that stood out.
There are a lot more people working on this problem now than twenty years ago. This is because of possible solar energy applications.
Diabatic states are the key to understanding. There are four relevant states. Simply the proton can be on the donor or acceptor. The electron can be on the donor or the acceptor. Whether the process is concerted or sequential depends on the relative energy of these four states.
A key question is whether the process is adiabatic or non-adiabatic.
What are the key experimental signatures of each?
One contrast is coupled electron-proton transfer (EPT) and hydrogen atom transfer (HAT).
The two cases are nicely embodied respectively in the model systems
HAT - benzyl/toluene
EPT - phenoxly/phenol
The theoretical details are worked out here.
In some enzymes such as soybean lipoxygenase (SLO) there are very large kinetic isotope effects (~80) for proton transfer, orders of magnitude larger than expected. Many people, including me, have struggled to understand this in terms of proton tunnelling in an adiabatic picture with coupling to an environment.
However, the relevant reactions are actually coupled electron-proton transfer, in the non-adiabatic regime. The key equation to understand both the magnitude and temperature dependence of the isotope effect is
taken from this paper.
A recent paper compares the theory to a mutant of SLO in which the isotope effect becomes ~500 as a result of the increase in the proton donor-acceptor distance R.
One minor point on how this relates to my talk. I said that quantum nuclear effects [and H/D isotope] effects were largest [and very subtle] in hydrogen bonding for donor-acceptor distances of R= 2.4-2.5 Angstroms. In contrast, here the isotope effects actually get larger with increasing R, with R=2.7 A for the wild-type SLO and increasing to 2.8-2.9 A with the selected mutations. I thank Sharon for pointing out this difference to me.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...
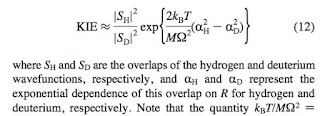



No comments:
Post a Comment