I would welcome any feedback on what I have done so far. Here is the draft of the abstract.
Emergent states of quantum matter
When a system is composed of many interacting components new properties can emerge that are qualitatively different from the properties of the individual components. Such emergent phenomena leads to a stratification of reality and of scientific disciplines.
Emergence can be particularly striking and challenging to understand for quantum matter, which is composed of macroscopic numbers of particles that obey quantum statistics. Examples included superfluidity, superconductivity, and the fractional quantum Hall effect. I will introduce some of the organising principles for describing
such phenomena: quasi-particles, spontaneously broken symmetry, and effective Hamiltonians. I will briefly describe how these ideas undergird some of my own research on complex molecular materials such as organic charge transfer salts, fluorescent proteins, and hydrogen bonded complexes. The interplay of emergence and reductionism raises issues in philosophy and as to the best scientific strategy for describing complex systems.
Let me know of any ways to make any of this clearer and more interesting.




On the integer QHE, do you mean "this quantisation holds exactly, up to experimental uncertainty of 10^-7" or "this quantisation is not exact, but is only 10^-7 off"? I assume it's the former?
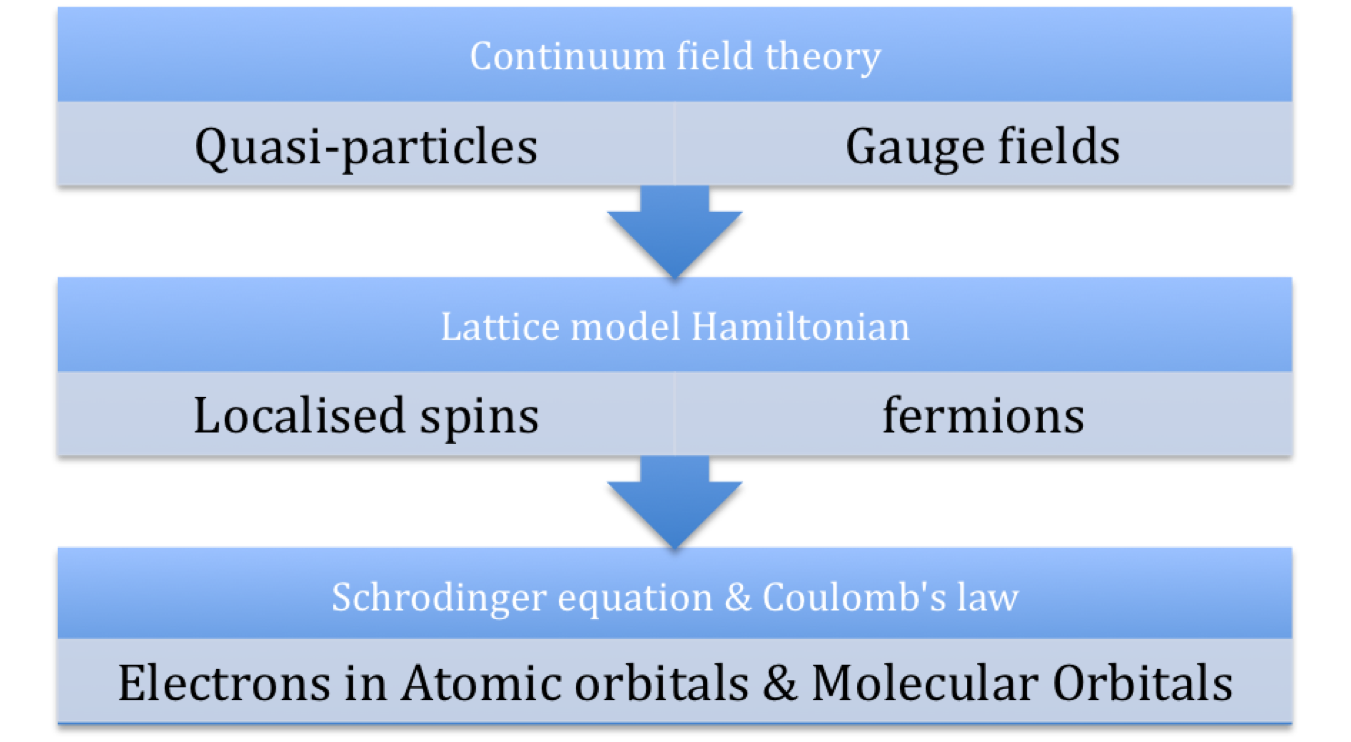
ReplyDeleteFor the minimal model effective Hamiltonian for k-(ET)2X, I would like to see more on the slide about what the terms mean. Whenever people write down a Hamiltonian with t and u and creation and annihilation operators, I always struggle to work out how everything relates to kinetic and potential energy, which are the only types of energy that exist. Those of us who don't work with second quantisation need a helping hand!
Thanks for the helpful feedback.
DeleteQuantum effect in liquid water may be an interesting example.
ReplyDeleteHi Ross, kudos for your effort in making it intelligible for people not on your field, I too am tired of sitting in talks that look like group seminars. That being said I do have a few comments
ReplyDeleteI'm very at odds with the comparison with geometry in page 3. From an axiomatic point of view the axioms for solid geometry are added to the ones for plane geometry, therefore there are a lot of diferent possibilities when adding dimensions. It is not that is difficuld to understand higher dimensional objects in terms of low dimensonal ones, it is impossible. A clear example is that line in an undefined concept in Hilbert's axioms, so is meaningless to say it is a collection of point with such and such properties. This is a very different situation from BCS theory for superconuctors I reckon. I'm not a condensed matter physicist, but I guess that emergence is a different question than the slides suggest, and more subtle than usually assumed. My point is that if I attended your seminar I would strongly object to such analogy, unless you stated that this analogy is strictly heuristical and should not be taken very seriously.
Also I know Anderson's article is paramout in emergence talks, but I'm disconcerted with his views of the so called stratification of disciplines. My experience reading chemists papers passed the impression that there is a difference in the frame which they view science, in that they re more concerned with rough guidelines for predicting reactions and compounds than with detailed mechanicsms, which is also at odds with your statements in page 11. I would say that such claims are very contentious and you should be prepared for lively discussions when taking questions after the talk, questions which can take rather philosophical twists in no time, though I guess you're well acquainted with that.
A final remark concerning the "theory of everything" in page 10, I guess you wrote "most of chemistry and solid state physics" with the hindsight that spin-orbit can be relevant, but I would like to mention a second example, namely relativistic effects in the periodic table, which lead to gold being yellow and mercury being liquid at room temperatures. The idea is that when you add more charge to the nucleus the s and p shell electrons have lots of energy and get to relativistic speeds, so you must take in account lorentz contraction of orbitals. I found this article very interesting on this topic http://pubs.acs.org/doi/abs/10.1021/ar50140a002, though I must say that sometimes the authors seem a bit arrogant. I juts find this a nice twist in "more is different" in that more protons in the nucleus lead to interesting different macroscopical phenomena, but not from low energy behavior.
Please don't take this as a critique of your presentation as such, I have most profited from reading your blog, even though I work in general relativity, it seems a very nice talk overall
Hi Cesar,
DeleteThanks for your thoughtful and helpful comments. I will certainly take them into account.
I see your point about the geometry analogy. I had not thought of that before. I now see that reductionism between levels does not quite work. But, it does illustrate how new concepts and properties emerge at higher levels.
I realise there are spin-orbit coupling (relativistic) effects that are important in chemistry and solid state that are not captured by the equation I show.
I will point out verbally this qualification.
Hopefully, the talk will generate some lively discussion of some of the issues you raise.