Are There Really Low-Barrier Hydrogen Bonds in Proteins? The Case of Photoactive Yellow Protein
Marc Nadal-Ferret, Ricard Gelabert, Miquel Moreno, and José M. Lluch
Low-barrier hydrogen bonds are characterised by an energy barrier to proton transfer that is comparable to the vibrational zero-point energy. As a consequence the proton is delocalised between the donor and the acceptor atoms.
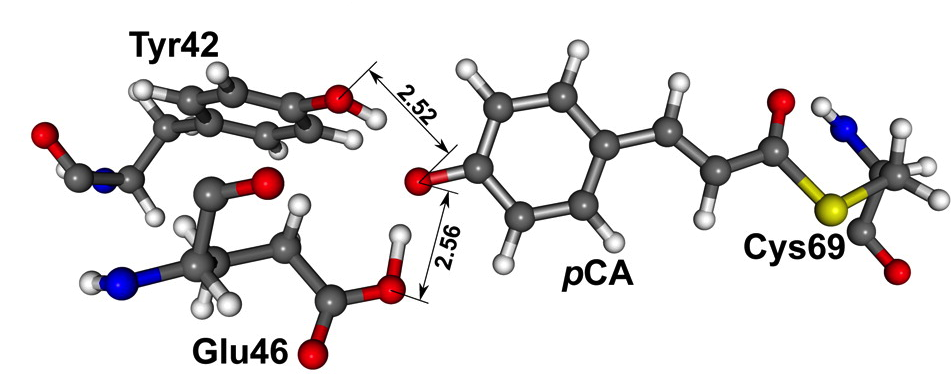
Previously I posted about the general issue of whether these bonds exist in proteins, and more importantly whether they have a functional role. Before I started working on H-bonds I wrote a post about new experimental studies claiming that the photoactive yellow protein has a low barrier H-bond (LBHB). The relevant geometry and the two relevant H-bonds [2.52 and 2.56 Angstroms] are shown below.
The key issue this JACS paper addresses is
in several very recent papers, Saito and Ishikita (33-35) have claimed that … the chemical properties of the pCA···Glu46 bond can be simply explained as a conventional hydrogen bond, without invoking the LBHB concept. In particular,(33) they have carried out quantum mechanical/molecular mechanical (QM/MM) calculations to reproduce the two short hydrogen bond distances of the crystal structure, obtaining 2.57 and 2.50 Å for pCA···Glu46 and pCA···Tyr42, respectively, but they have not found any minimum energy structure with the proton near the central region of the hydrogen bonds. In both cases, the electronic structure calculations lead to energy minima with the two protons clearly belonging to the Glu46 or Tyr42 moieties, respectively.However, as the authors stress, this earlier work treats the proton classically. The JACS takes into account quantum effects of the proton motion.
They find a low-dimensional ground state potential energy surface using several QM/MM methods [most DFT with the CAM-B3LYP functional] and then find the low-lying vibrational eigenstates, for both protons and deuterium. They conclude
our work supports the dual result that, in the solid (crystal) phase, PYP presents an LBHB in the pCA···Glu46 hydrogen bond, whereas in solution this strong interaction is gone and shows characteristics of a “normal” hydrogen bond, much in line with what was found for many simpler systems by Perrin et al. (26, 27) Then our results support the first direct experimental demonstration of the formation of an LBHB in a protein.I am wondering how robust these results are. There are some extreme subtleties.
First, the results for the potential energy surface for proton transfer may depend significantly on the quantum chemical method that is used and on the method for dividing the QM/MM region.
Second, following some of the results in my recent preprint, when the donor-acceptor distance R is about 2.5 Angstroms (A) quantum effects become very significant leading to
- an increase in the average value of R from the minimum of the classical potential by about 0.1 A due to zero-point energy of the proton.
- a difference of about 0.05 A between hydrogen and deuterium. This is significant because it means the distances determined from neutron scattering on deuterated crystals will be different from the native protein with protons.
- a significant change in the proton transfer potential [particularly the size of the energy barrier] as R changes by amounts as small as 0.05 A.
Due to all of the above I think that it is going to be difficult to make definitive conclusions about this fascinating and important issue.




No comments:
Post a Comment