Today I am giving a CMT seminar in the Physics Department at the Indian Institute of Science in Bangalore, "Spin frustration in organic Mott insulators: from quantum spin liquids to superconductors." Slides are here.
The talk material is covered in great detail in a review article, written with Ben Powell.
Thursday, January 31, 2013
Wednesday, January 30, 2013
Postdoc available in condensed matter theory at UQ
I have just advertised for a new postdoc to work with me on the theory of strongly correlated electron materials. You can see more details via the official advertisement. The flavour of some of the research can be seen from my blog posts under the "thermoelectric" label on this blog.
Tuesday, January 29, 2013
Talk on quantum proton transfer in enzymes
On wednesday morning I am giving an informal talk, Quantum proton transfer in enzymes, in the Dept. of Inorganic and Physical Chemistry at IISc Bangalore.
It is largely based on this paper.
It is largely based on this paper.
Monday, January 28, 2013
Dramatic isotope effects near the Mott insulator
When I gave a seminar last week in the Inorganic and Physical Chemistry department at IISc the slide that generated the most interest and discussion was the one below.
The figure is taken from this PRB.
It shows how as one increases the number of deuterium atoms (replacing H) on the BEDT-TTF molecules (shown below) one gradually moves from the superconducting (S.C.) phase into the antiferromagnetic Mott insulator (A.F.I) phase.
This transition is analogous to what happens if one decreases the pressure or replaces the Br with Cl, i.e. it is generally associated with a change in the intermolecular spacings. Theory suggests this corresponds to increasing U/W [ratio of the Hubbard U to the bandwidth W]. It may also be due to a decrease in electronic frustration.
Why is this surprising?
First, this must be a quantum nuclear effect (e.g. due to zero point motion) because the chemical forces [potential energy surfaces] for H and D are identical. This is the Born-Oppenheimer approximation.
Second, the relevant molecular orbitals are centred [i.e. have the dominant electronic density] on the S and C atoms and not the H atoms.
Third, one can expect some sort of geometric isotope effect where there are small changes in bond lengths and crystal lattice parameters with H to D substitution. However, generally these effects are very small, particularly for systems in which hydrogen bonding plays a small role. Here the H bonding is with the anion. Thus it is hard to see how these small geometric changes might produce large enough changes in the band structure parameters t and t' in the relevant Hubbard model.
Further insight might be gained by doing DFT-based calculations of the parameters t and t' for the geometries of the H and D crystals determined by x-ray diffraction.
The figure is taken from this PRB.
It shows how as one increases the number of deuterium atoms (replacing H) on the BEDT-TTF molecules (shown below) one gradually moves from the superconducting (S.C.) phase into the antiferromagnetic Mott insulator (A.F.I) phase.
This transition is analogous to what happens if one decreases the pressure or replaces the Br with Cl, i.e. it is generally associated with a change in the intermolecular spacings. Theory suggests this corresponds to increasing U/W [ratio of the Hubbard U to the bandwidth W]. It may also be due to a decrease in electronic frustration.
Why is this surprising?
First, this must be a quantum nuclear effect (e.g. due to zero point motion) because the chemical forces [potential energy surfaces] for H and D are identical. This is the Born-Oppenheimer approximation.
Second, the relevant molecular orbitals are centred [i.e. have the dominant electronic density] on the S and C atoms and not the H atoms.
Third, one can expect some sort of geometric isotope effect where there are small changes in bond lengths and crystal lattice parameters with H to D substitution. However, generally these effects are very small, particularly for systems in which hydrogen bonding plays a small role. Here the H bonding is with the anion. Thus it is hard to see how these small geometric changes might produce large enough changes in the band structure parameters t and t' in the relevant Hubbard model.
Further insight might be gained by doing DFT-based calculations of the parameters t and t' for the geometries of the H and D crystals determined by x-ray diffraction.
Friday, January 25, 2013
Geometric solid state chemistry
A while ago I posted about the incredibly rich crystal structure of boron which has a unit cell of hundreds of atoms.
Today at IISER-TVM Jemmis gave me a nice overview of his work [see this paper] showing how this and the associated defect structures emerge naturally from several building blocks including B_12 icosahedra which turn out to be particularly stable.
One call also make fruitful analogies with Huckel type rules associated with aromatic molecules such as benzene.
Today at IISER-TVM Jemmis gave me a nice overview of his work [see this paper] showing how this and the associated defect structures emerge naturally from several building blocks including B_12 icosahedra which turn out to be particularly stable.
One call also make fruitful analogies with Huckel type rules associated with aromatic molecules such as benzene.
Wednesday, January 23, 2013
Hydrogen bonding in Kerala
On thursday I am visiting the new Indian Institute of Science Education and Research Thiruvananthapuram (IISER-TVM) [Trivandrum, Kerala], an hours flight from Bangalore.
I will give a talk to a mixed chemistry-physics audience,
"A quantum physicist looks at hydrogen bonding."
Here are the slides of the current version.
It is largely based on this paper.
I recently posted earlier about some nice work my host Jemmis did on blue-shifted hydrogen bonds.
I will give a talk to a mixed chemistry-physics audience,
"A quantum physicist looks at hydrogen bonding."
Here are the slides of the current version.
It is largely based on this paper.
I recently posted earlier about some nice work my host Jemmis did on blue-shifted hydrogen bonds.
From chemistry to RVB and spin liquids
Here are the slides for my talk this afternoon in the Department of Inorganic and Physical Chemistry at the Indian Institute of Science in Bangalore.
Superconducting organic charge transfer salts: from chemistry to quantum many-body physics
I feel the talk does not quite flow and needs work but I have run out of time so will see how it goes. One can't be a perfectionist, particularly the first time one gives a specific talk. You also have to be careful when making visits you does not spend most of your time preparing your talk, rather than actually talking science with your hosts!
Superconducting organic charge transfer salts: from chemistry to quantum many-body physics
I feel the talk does not quite flow and needs work but I have run out of time so will see how it goes. One can't be a perfectionist, particularly the first time one gives a specific talk. You also have to be careful when making visits you does not spend most of your time preparing your talk, rather than actually talking science with your hosts!
Tuesday, January 22, 2013
Anderson conceived RVB in Bangalore
In preparing my talk for tomorrow in Bangalore I remembered that Phil Anderson's seminal 1987 Science paper, The Resonating Valence Bond State in La2Cu2O4 and superconductivity has the following acknowledgement.
Also on the subject of the history Anderson discusses it more in his recent book, including how excited he was on the flight back from India.
In Physics Today he wrote a short Reference Frame Who or What is RVB? which describes the inspiration in the 1970s from Pauling's chemical ideas.
This is an important example of the fruitful exchange of ideas between chemistry and physics. Note that the flow is not just in one direction!
Saturday, January 19, 2013
Are international branch campuses problematic?
When I was in Sri Lanka I was intrigued to see a few obscure universities from Australia and the UK were setting up programs offering degrees there. Of course the large advertising billboards claimed how "world class" they were.
At the other end of the prestige scale, I was interested to read an article in the Economist Foreign Universities find working in China harder than they expected. It points out how Yale abandoned a joint program with Peking University. One of the reasons may have been concerns about rampant plagiarism.
A number of Australian universities have lost serious money (and face) in failed ventures in Asia. Some of the issues are discussed here.
One should never under-estimate two very powerful forces
- culture
- financial greed.
The latter tends to lead to wishful thinking which overlooks the role of the first.
At the other end of the prestige scale, I was interested to read an article in the Economist Foreign Universities find working in China harder than they expected. It points out how Yale abandoned a joint program with Peking University. One of the reasons may have been concerns about rampant plagiarism.
A number of Australian universities have lost serious money (and face) in failed ventures in Asia. Some of the issues are discussed here.
One should never under-estimate two very powerful forces
- culture
- financial greed.
The latter tends to lead to wishful thinking which overlooks the role of the first.
Friday, January 18, 2013
Something more to worry about
This week in Science there are some interesting and worrying articles showing and discussing that Genealogy Databases Enable Naming of Anonymous DNA Donors
Thursday, January 17, 2013
Abstract audiences
I have posted before that a key to attracting an audience to a talk is to write an effective and engaging abstract that will motivate the target audience to attend. The abstract (talk) will be different if the target audience is chemists or physicists, experimentalists or theorists, experts or non-experts.
I was invited to give a seminar in the Department of Inorganic and Physical Chemistry in the Indian Institute of Science at Bangalore. My hosts asked for a general talk that might also attract people from the Physics department and the Solid State and Structural Chemistry Unit.
Here is the abstract I composed.
Superconducting organic charge transfer salts: from chemistry to quantum many-body physics
A recent essay suggested that chemistry research in India could benefit from a greater interaction with physics [1].
I will give an example of an exciting research field where there has been a rich interaction between physics and chemistry.
Charge transfer salts based on molecules such as bis(ethylenedithio)tetrathiafulvalene [BEDT-TTF] and 1,3-dithiole-2-thione-4,5-dithiolate [dmit] exhibit a rich chemistry.
Small chemical substitutions in the charge donor or acceptor lead to changes in crystal structure due to the subtle interplay of covalent, ionic, and hydrogen bonding. Furthermore, these small structural changes can lead to qualitative changes in physical properties. In particular, one can tune between insulating, metallic, and superconducting states.
Over the past five years these materials have attracted significant new attention from physicists because their Mott insulating phase can exhibit long sought after exotic
quantum many-body ground states including a spin liquid and valence bond crystal [2]. Furthermore, application of pressure can induce a transition into an unconventional superconducting state. Consequently, these materials realise seminal ideas of Anderson [3] concerning resonating valence bonds and superconductivity.
I will emphasize some of the rich interchange of ideas between chemistry and physics.
Some of the cultural and institutional obstacles to collaboration between chemistry and physics will also be discussed.
[1] E. Arunan, R. Brakaspathy, G.R. Desiraju, and S. Sivaram, Angew. Chem. Int. Ed. 52, 114 (2013).
[2] B.J. Powell and R.H. McKenzie, Reports of Progress in Physics 74, 056501 (2011).
[3] P.W. Anderson, Science 235, 1196 (1987).
The seminar is next wednesday. I will post the slides once I have prepared the talk!
I was invited to give a seminar in the Department of Inorganic and Physical Chemistry in the Indian Institute of Science at Bangalore. My hosts asked for a general talk that might also attract people from the Physics department and the Solid State and Structural Chemistry Unit.
Here is the abstract I composed.
Superconducting organic charge transfer salts: from chemistry to quantum many-body physics
A recent essay suggested that chemistry research in India could benefit from a greater interaction with physics [1].
I will give an example of an exciting research field where there has been a rich interaction between physics and chemistry.
Charge transfer salts based on molecules such as bis(ethylenedithio)tetrathiafulvalene [BEDT-TTF] and 1,3-dithiole-2-thione-4,5-dithiolate [dmit] exhibit a rich chemistry.
Small chemical substitutions in the charge donor or acceptor lead to changes in crystal structure due to the subtle interplay of covalent, ionic, and hydrogen bonding. Furthermore, these small structural changes can lead to qualitative changes in physical properties. In particular, one can tune between insulating, metallic, and superconducting states.
Over the past five years these materials have attracted significant new attention from physicists because their Mott insulating phase can exhibit long sought after exotic
quantum many-body ground states including a spin liquid and valence bond crystal [2]. Furthermore, application of pressure can induce a transition into an unconventional superconducting state. Consequently, these materials realise seminal ideas of Anderson [3] concerning resonating valence bonds and superconductivity.
I will emphasize some of the rich interchange of ideas between chemistry and physics.
Some of the cultural and institutional obstacles to collaboration between chemistry and physics will also be discussed.
[1] E. Arunan, R. Brakaspathy, G.R. Desiraju, and S. Sivaram, Angew. Chem. Int. Ed. 52, 114 (2013).
[2] B.J. Powell and R.H. McKenzie, Reports of Progress in Physics 74, 056501 (2011).
[3] P.W. Anderson, Science 235, 1196 (1987).
The seminar is next wednesday. I will post the slides once I have prepared the talk!
Tuesday, January 15, 2013
What is the future of Indian science?
This next few weeks I am visiting the Indian Institute of Science in Bangalore. My host Arunan recently co-authored a stimulating essay Chemistry in India: Unlocking the Potential in Angewandte Chemie.
It seems to me that much of the analysis may also be relevant to physics in India. Perhaps, someone would like to comment.
The decade since 2000 has seen exponential growth in investments in research and education. Research expenditure nearly doubled from $ 12.9 billion in 2002 to $ 24.8 billion in 2007, and further to $ 41.3 billion in 2012 (on the basis of purchasing power parity). Around 50 new universities and institutions have been started. These include 5 Indian Institutes of Science Education and Research, 9 Indian Institutes of Technology, 16 Central Universities, and several National Institutes of Technology, and National Institutes of Pharmaceutical Education and Research.The authors critically examine whether all this investment, which should certainly be celebrated, will translate into research excellence. They are frank about some of the cultural obstacles, particularly fear of taking risks and crossing disciplinary boundaries.
It seems to me that much of the analysis may also be relevant to physics in India. Perhaps, someone would like to comment.
Monday, January 14, 2013
Deconstructing excited state dynamics in a solvent
A key question about excited state dynamics in a solvent is the relative importance of the solvent polarity and the viscosity.
This is examined experimentally in the paper
Ultrafast Photoisomerization of Photoactive Yellow Protein Chromophore Analogues in Solution: Influence of the Protonation State
Agathe Espagne, Daniel Paik, Pascale Changenet-Barret, Monique Martin, Ahmed H. Zewail
The photoisomerization corresponds to the twisting of the double bond shown below.
The figure above shows the de-protonated (anionic) form. The protonated (neutral) form has a proton attached to the oxygen anion on the right end of the molecule.
They find that the excited state lifetime of the protonated (de-protonated) chromophore varies significantly with the solvent viscosity (polarity) but not the polarity (viscosity).
It is not surprising to me that the shape of the excited state potential energy surface varies significantly with the protonation state. Seth Olsen has shown this clearly for the chromophore of the green fluorescent protein in high level quantum chemistry calculations. These surfaces can be described by a simple two-state effective Hamiltonian (see here). The key physics is that de-protonation tunes the system away from "resonance" between the two underlying valence bond states. Generally, when one is close to resonance the ground and excited state have small net dipole moments and one expects a weak coupling to the polarity of the solvent.
A key challenge is constructing the simplest possible effective Hamiltonian which can describe the excited state dynamics including the effective of the solvent viscosity and polarity.
This is examined experimentally in the paper
Ultrafast Photoisomerization of Photoactive Yellow Protein Chromophore Analogues in Solution: Influence of the Protonation State
Agathe Espagne, Daniel Paik, Pascale Changenet-Barret, Monique Martin, Ahmed H. Zewail
The photoisomerization corresponds to the twisting of the double bond shown below.
The figure above shows the de-protonated (anionic) form. The protonated (neutral) form has a proton attached to the oxygen anion on the right end of the molecule.
They find that the excited state lifetime of the protonated (de-protonated) chromophore varies significantly with the solvent viscosity (polarity) but not the polarity (viscosity).
It is not surprising to me that the shape of the excited state potential energy surface varies significantly with the protonation state. Seth Olsen has shown this clearly for the chromophore of the green fluorescent protein in high level quantum chemistry calculations. These surfaces can be described by a simple two-state effective Hamiltonian (see here). The key physics is that de-protonation tunes the system away from "resonance" between the two underlying valence bond states. Generally, when one is close to resonance the ground and excited state have small net dipole moments and one expects a weak coupling to the polarity of the solvent.
A key challenge is constructing the simplest possible effective Hamiltonian which can describe the excited state dynamics including the effective of the solvent viscosity and polarity.
Friday, January 11, 2013
Should you teach this proposed new course?
Probably not.
Universities and colleges love introducing new courses, whether it is "Financial mathematics", "Bioinformatics", "Philosophy of Physics" or "Current trends in nanotechnology"?
Should un-tenured faculty get involved in these enterprises?
Over the years I have seen many such ventures and been involved in some at a range of institutions. Often these proposals are driven by a departments desire to boost student numbers, particularly by attracting students from other majors and programs. Thus, many of the offerings are cross-disciplinary and so involve more than one department.
Often attempts are made to recruit un-tenured faculty or research staff to help teach or start these courses, with a vague promise that if it goes well there may be a permanent job associated with it.
There is a simple question that can be asked to determine whether or not you should get involved:
Is there any department or major which will make it compulsory for their students to take the course?
To make this explicit. For a course in financial mathematics: will it be compulsory for mathematics or finance/economics/business majors?
If the answer is yes. Then the course may be viable.
Usually the answer is no.
This may mean the course will struggle to attract significant student numbers. It will not be a big money (or goodwill) generator for your department. The course may then gradually die out. Furthermore, who will be to blame? Not, the senior faculty member who enthusiastically proposed the course. Rather, probably the junior faculty who spent all the time developing it and teaching it to small classes.
Universities and colleges love introducing new courses, whether it is "Financial mathematics", "Bioinformatics", "Philosophy of Physics" or "Current trends in nanotechnology"?
Should un-tenured faculty get involved in these enterprises?
Over the years I have seen many such ventures and been involved in some at a range of institutions. Often these proposals are driven by a departments desire to boost student numbers, particularly by attracting students from other majors and programs. Thus, many of the offerings are cross-disciplinary and so involve more than one department.
Often attempts are made to recruit un-tenured faculty or research staff to help teach or start these courses, with a vague promise that if it goes well there may be a permanent job associated with it.
There is a simple question that can be asked to determine whether or not you should get involved:
Is there any department or major which will make it compulsory for their students to take the course?
To make this explicit. For a course in financial mathematics: will it be compulsory for mathematics or finance/economics/business majors?
If the answer is yes. Then the course may be viable.
Usually the answer is no.
This may mean the course will struggle to attract significant student numbers. It will not be a big money (or goodwill) generator for your department. The course may then gradually die out. Furthermore, who will be to blame? Not, the senior faculty member who enthusiastically proposed the course. Rather, probably the junior faculty who spent all the time developing it and teaching it to small classes.
One of the biggest challenges of teaching
Accept the students in your class as they are.
They are given to you with their limited abilities, background knowledge, skills, and training.
We often wish that they were smarter or better prepared.
If we don't accept students and work with them as they are there are several things that can happen:
1. We can waste a lot of emotional energy complaining about the quality of our students. "In the good old days .... " or "When I was a student ...."
2. Frustration with the students can create emotional distance (even hostility) that reduces the effectiveness of our teaching.
3. We plough on with our planned curriculum, level of course, and high standards. Most of the students then learn almost nothing. Our goal should be to move them one step further in their education, ... for them to just learn something.
For example, in third year physics it may be making sure that they finally learn what we think they should have learnt in first year [e.g. how to work with physical units].
This may require adjustment of our plans and expectations.
There may be legitimate issues about pre-requisites, admission standards, or high school curricula that need to be addressed to improve the quality of the students we teach. However, once the class starts all that is irrelevant. There is nothing we can do about it.
Those issues are to be dealt with in a different forum.
They are given to you with their limited abilities, background knowledge, skills, and training.
We often wish that they were smarter or better prepared.
If we don't accept students and work with them as they are there are several things that can happen:
1. We can waste a lot of emotional energy complaining about the quality of our students. "In the good old days .... " or "When I was a student ...."
2. Frustration with the students can create emotional distance (even hostility) that reduces the effectiveness of our teaching.
3. We plough on with our planned curriculum, level of course, and high standards. Most of the students then learn almost nothing. Our goal should be to move them one step further in their education, ... for them to just learn something.
For example, in third year physics it may be making sure that they finally learn what we think they should have learnt in first year [e.g. how to work with physical units].
This may require adjustment of our plans and expectations.
There may be legitimate issues about pre-requisites, admission standards, or high school curricula that need to be addressed to improve the quality of the students we teach. However, once the class starts all that is irrelevant. There is nothing we can do about it.
Those issues are to be dealt with in a different forum.
Wednesday, January 9, 2013
What is your dream referee report?
The referee reports you get back from journals can vary greatly in quality, length, tone, and character.
Here are some broad types. I have received all types over the years.
1. Publish paper as is. A few generic positive comments about the paper.
2. Publish the paper once the following issues have been addressed.
A long and detailed list of criticisms, suggestions, questions, and comments.
3. Send to another journal. Paper is not "important" for vague subjective reasons. No detailed criticism or discussion.
4. Send to another journal. Paper is not "important" for subjective reasons.
A long and detailed list of criticisms, suggestions, questions, and comments.
5. Reject. Superficial criticisms.
Most people might say that 1. is their dream. It certainly makes life easy. No further work is required. You get to add another line to your CV and focus on the next publon. But, did the referees actually read the paper and engage with the scientific content? If they did not will anyone else?
2. is my dream. It shows that the referees thought the paper was interesting and important enough to take the time and make the effort to read it carefully, think about it, and write out ways it could be improved. To me this is high praise.
Very rarely have I received such reports.
Does that say something about the quality of my papers or the quality of refereeing?
Seth Olsen and I did get such reports for this recent J. Chem. Phys. I was really encouraged.
But, all this constructive criticism requires more work to address.
4. Is less desirable than 1. for career advancement but is in some ways represents higher scientific praise.
What has been your experience?
Here are some broad types. I have received all types over the years.
1. Publish paper as is. A few generic positive comments about the paper.
2. Publish the paper once the following issues have been addressed.
A long and detailed list of criticisms, suggestions, questions, and comments.
3. Send to another journal. Paper is not "important" for vague subjective reasons. No detailed criticism or discussion.
4. Send to another journal. Paper is not "important" for subjective reasons.
A long and detailed list of criticisms, suggestions, questions, and comments.
5. Reject. Superficial criticisms.
Most people might say that 1. is their dream. It certainly makes life easy. No further work is required. You get to add another line to your CV and focus on the next publon. But, did the referees actually read the paper and engage with the scientific content? If they did not will anyone else?
2. is my dream. It shows that the referees thought the paper was interesting and important enough to take the time and make the effort to read it carefully, think about it, and write out ways it could be improved. To me this is high praise.
Very rarely have I received such reports.
Does that say something about the quality of my papers or the quality of refereeing?
Seth Olsen and I did get such reports for this recent J. Chem. Phys. I was really encouraged.
But, all this constructive criticism requires more work to address.
4. Is less desirable than 1. for career advancement but is in some ways represents higher scientific praise.
What has been your experience?
Tuesday, January 8, 2013
A possible mechanism for longitudinal magnetoresistance
I have written several posts (e.g, this post) about the outstanding question of the origin of the longitudinal interlayer magnetoresistance is some layered metals. They have the strange property that the magnetoresistance is largest (smallest) when the current is parallel (perpendicular) to the magnetic field direction. This is the opposite angular dependence to that expected if the Lorentz force (F=qvxB) causes the magnetoresistance, as it does in most metals.
Tony Wright brought to my attention a recent preprint
Longitudinal interlayer magnetoresistance in quasi-2D metals
P. D. Grigoriev
He finds a magnetoresistance which has the desired properties. Basically, the magnetoresistance arises because electron scattering rate becomes dependent on the magnetic field. He considers the Landau level structure and calculates the self-energy according to the self-consistent Born approximation for scattering from point-like impurities.
Considering the relative magnitude of the different energy scales
t_perp (interlayer hopping integral), hbar omega_c (Landau level spacing), Gamma (Landau level width due to impurities), Fermi energy
is key to the analysis.
As the magnetic field increases there is crossover from a linear dependence on the magnitude of the magnetic field perpendicular to the layers to a square root dependence.
An outstanding question is whether this mechanism can also describe the unusual temperature dependence, including the violation of Kohler's rule, that is usually associated with this magnetoresistance.
Tony Wright brought to my attention a recent preprint
Longitudinal interlayer magnetoresistance in quasi-2D metals
P. D. Grigoriev
He finds a magnetoresistance which has the desired properties. Basically, the magnetoresistance arises because electron scattering rate becomes dependent on the magnetic field. He considers the Landau level structure and calculates the self-energy according to the self-consistent Born approximation for scattering from point-like impurities.
Considering the relative magnitude of the different energy scales
t_perp (interlayer hopping integral), hbar omega_c (Landau level spacing), Gamma (Landau level width due to impurities), Fermi energy
is key to the analysis.
As the magnetic field increases there is crossover from a linear dependence on the magnitude of the magnetic field perpendicular to the layers to a square root dependence.
An outstanding question is whether this mechanism can also describe the unusual temperature dependence, including the violation of Kohler's rule, that is usually associated with this magnetoresistance.
Monday, January 7, 2013
Strongly correlated toplogical insulators
At the Journal Club for Condensed Matter Chandra Varma has a helpful commentary on
recent experimental papers reporting evidence that the mixed valence compound SmB6 is a topological insulator (see my earlier post and a recent Nature News article).
A few things I learnt from Varma's commentary.
The strongly correlated properties are not central to the topological properties. These are rather a property of the effective band structure which arises in a slave boson treatment or the Varma-Yafet variational wavefunction.
The Fu-Kane conditions for a topological insulator most likely hold in the mixed valence limit which is at the extreme of particle-hole asymmetry. In this limit the Kondo temperature is of the order of the hybridisation energy, in contrast to the Kondo limit when it is an order of magnitude smaller.
Some caution is in order because the existence of actual surface states [e.g. from ARPES] have not yet been definitively established.
Definitive signatures of the strongly correlated state might be seen in new low energy resonances that could arise from non-magnetic impurity substitution.
p.s. On his website Piers Coleman has a nice talk giving the background theory which preceded the experiments.
recent experimental papers reporting evidence that the mixed valence compound SmB6 is a topological insulator (see my earlier post and a recent Nature News article).
A few things I learnt from Varma's commentary.
The strongly correlated properties are not central to the topological properties. These are rather a property of the effective band structure which arises in a slave boson treatment or the Varma-Yafet variational wavefunction.
The Fu-Kane conditions for a topological insulator most likely hold in the mixed valence limit which is at the extreme of particle-hole asymmetry. In this limit the Kondo temperature is of the order of the hybridisation energy, in contrast to the Kondo limit when it is an order of magnitude smaller.
Some caution is in order because the existence of actual surface states [e.g. from ARPES] have not yet been definitively established.
Definitive signatures of the strongly correlated state might be seen in new low energy resonances that could arise from non-magnetic impurity substitution.
p.s. On his website Piers Coleman has a nice talk giving the background theory which preceded the experiments.
Saturday, January 5, 2013
Some challenges of science in the developing world
I spent yesterday talking to staff of the chemistry and physics departments at the University of Colombo in Sri Lanka.
Last year academic staff at all Sri Lanka universities were on strike for several months demanding greater government investment in education. [See this article from the Economist].
The challenges of teaching science are formidable. These challenges include:
Last year academic staff at all Sri Lanka universities were on strike for several months demanding greater government investment in education. [See this article from the Economist].
The challenges of teaching science are formidable. These challenges include:
- The poor English of students, particularly those from rural areas.
- The limited availability and ongoing maintenance of even basic lab equipment such as pH meters and mass balances.
- Complex government and bureaucratic rules and regulations for importing chemicals, spare parts, and equipment. So even on the rare occasions when funds are available actually acquiring the desired equipment or consumables can be problematic.
- A lack of fundamental understanding of the basics, even from students have performed extremely well on Cambridge A-levels exams. It seems some students prepare for these exams by just memorisation of answers to past exam questions.
- Poor attendance at lectures.
- Limited instrumentation for even basic chemical and structural characterisation of samples.
- Limited (or very slow) internet access for both students and staff.
In spite of all these obstacles staff are proud of the job training their students. Each year about half of their graduates go to Ph.D programs in the USA.
Australia is considered a less desirable destination because of the limited funding it offers to international Ph.D students and the absence of graduate level course work.
Staff also persevere with research, somehow managing to publish papers in international journals. Often this relies on international collaborations [usually via the extensive network of expatriates in the West] to access instrumentation.
Unfortunately, few Ph.D graduates return to Sri Lanka, preferring to take jobs in US industry. There are many vacant faculty positions in Sri Lanka universities. The salary of a Full Professor was recently increased to slightly more than US$1,000 per month.
Thursday, January 3, 2013
Do good science or perish!
Today on a Brisbane-Singapore flight I read most of an interesting booklet Publishing Scientific Papers in the Developing World stemming from a 2010 conference.
Some of it is also relevant to the Western/North/Developed/Rich world as well.
Here are a few highlights.
Erik Thulstrup has a nice chapter "How should a Young Researcher Write and Publish a Good Research Paper?"
He points out that for young scientists, particularly those without experienced mentors, attempting to publish in good journals can provide valuable feedback about their research and writing. This is preferable to the easier route of publishing lots of papers in mediocre journals as a means to pad a resume.
Thulstrup also has a paper "Why Smaller Journals Should Merge" which describes the plethora of small inefficient journals in the developing world. These don't disseminate research but hide it!
There is a need to follow the example of Nordic countries from the 1960s to 1980s which saw many local and national journals merge and morph into effective international journals.
There is a helpful overview "Open Access" by Ramy Azis and Peter Binfield.
It describes the goals of PLOS (Public Library of Science).
It points out that Eugene Garfield, inventor of the Impact Factor, stressed it was mostly for librarians and that it should not be used to evaluate the value of individual papers.
The article ends with the dream that "publish or perish" may be replaced with "do good science or perish"
Some of it is also relevant to the Western/North/Developed/Rich world as well.
Here are a few highlights.
Erik Thulstrup has a nice chapter "How should a Young Researcher Write and Publish a Good Research Paper?"
He points out that for young scientists, particularly those without experienced mentors, attempting to publish in good journals can provide valuable feedback about their research and writing. This is preferable to the easier route of publishing lots of papers in mediocre journals as a means to pad a resume.
Thulstrup also has a paper "Why Smaller Journals Should Merge" which describes the plethora of small inefficient journals in the developing world. These don't disseminate research but hide it!
There is a need to follow the example of Nordic countries from the 1960s to 1980s which saw many local and national journals merge and morph into effective international journals.
There is a helpful overview "Open Access" by Ramy Azis and Peter Binfield.
It describes the goals of PLOS (Public Library of Science).
It points out that Eugene Garfield, inventor of the Impact Factor, stressed it was mostly for librarians and that it should not be used to evaluate the value of individual papers.
The article ends with the dream that "publish or perish" may be replaced with "do good science or perish"
Small is not beautiful
I am wondering if there is a scaling relation between the size of a grant and the administrative overhead/workload associated with it. My limited experience is that the relevant exponent is much less than one.
Small grants (especially travel grants of a few thousand $) seem to require comparable administration (pages of application, contract, memoranda of "understanding", special visas, progress reports, final reports, press releases, ....) as grants of several hundred thousand $. This administrative overhead is not just a burden on the scientist but wastes the money of the funding agency and university who are paying support staff to administer the grant.
Due to this large admin overhead I generally don't bother with small grants. However, I recently did successfully apply for one, partly for political and kudos reasons, and have been really struck by the problem.
Small grants (especially travel grants of a few thousand $) seem to require comparable administration (pages of application, contract, memoranda of "understanding", special visas, progress reports, final reports, press releases, ....) as grants of several hundred thousand $. This administrative overhead is not just a burden on the scientist but wastes the money of the funding agency and university who are paying support staff to administer the grant.
Due to this large admin overhead I generally don't bother with small grants. However, I recently did successfully apply for one, partly for political and kudos reasons, and have been really struck by the problem.
Wednesday, January 2, 2013
Edge states define the bulk
Until thirty years ago the boundary of a quantum system was just considered an annoying irrelevance that one wanted to get rid of [or make as small as possible] so one could focus on the bulk properties.
However, the fractional quantum Hall effect and more recently topological insulators have shown that the boundary [edge] can actually tell us something fundamental about the bulk and is interesting in its own right.
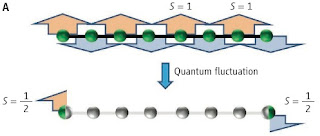
There is a nice Perspective in Science Symmetry meets topology by Xiao-Ling Qi. It introduces recent work by Xiao-Gang Wen and collaborators. They have used cohomology to classify symmetry protected topological states. A nice example is provided by the Haldane spin-1 antiferromagnetic chain. The bulk has an energy gap, but it has the highly non-trivial property that a finite chain has spin-1/2 excitations at the ends [This PRL reports experimental evidence]. Hence, the edges characterise the unusual properties of the system.
Wen also has a helpful review on the arXiv which provides a gentle introduction to topological order. It is fascinating, but to me it highlights that in two dimensions we are still a long way from clear material realisations and definitive experimental signatures.
However, the fractional quantum Hall effect and more recently topological insulators have shown that the boundary [edge] can actually tell us something fundamental about the bulk and is interesting in its own right.
There is a nice Perspective in Science Symmetry meets topology by Xiao-Ling Qi. It introduces recent work by Xiao-Gang Wen and collaborators. They have used cohomology to classify symmetry protected topological states. A nice example is provided by the Haldane spin-1 antiferromagnetic chain. The bulk has an energy gap, but it has the highly non-trivial property that a finite chain has spin-1/2 excitations at the ends [This PRL reports experimental evidence]. Hence, the edges characterise the unusual properties of the system.
Wen also has a helpful review on the arXiv which provides a gentle introduction to topological order. It is fascinating, but to me it highlights that in two dimensions we are still a long way from clear material realisations and definitive experimental signatures.
Subscribe to:
Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...