The dynamics of the atomic motion associated with most chemical reactions is classical. In particular, the rate of reaction is determined by the rate of thermal excitation over an energy barrier associated with the transition state (a key concept): a particular nuclear configuration which is a saddle point on the potential energy surface which contains both the reactants and products.
It is hard to find exceptions to this paradigm, e.g., where quantum tunneling below the barrier is important. Some people claim this picture breaks down for enzymes, as discussed in an earlier post. But I remain to be convinced, particularly that enzymes have evolved to make use of quantum tunneling.
However, I am convinced and fascinated by an article which does discuss some concrete exceptions to transition state theory for small molecules that have recently been discovered. Tunneling does not just lead to quantitative changes in reaction rates but different products of the chemical reaction.
There is a nice review of this work:
Tunnelling control of chemical reactions – the organic chemist’s perspective
David Ley, Dennis Gerbig and Peter R. Schreiner
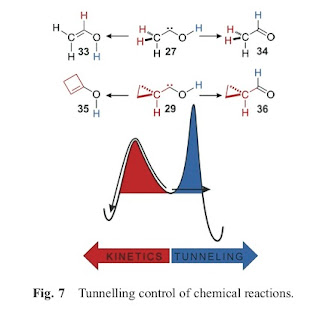
One of the main points is summarised in the figure below. If one starts with the molecule in the centre then at high temperatures the reaction proceeds to the left, because that product involves the lowest energy barrier (activation energy).
However, the energy barrier to produce the molecules shown on the right is narrower. Hence, when the reaction is dominated by tunneling (i.e. at low temperatures) one gets a different product.
Subscribe to:
Post Comments (Atom)
A forgotten physicist: Amelia Frank (1906-1937)
In honour of International Women's Day, I bring to your attention a fascinating recent piece in The Conversation , Who was Amelia Frank?...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



Great paper! Thanks for pointing it out.
ReplyDelete