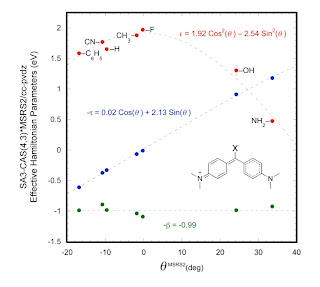
Seth Olsen and I have just finished a paper An Effective Hamiltonian for Symmetric Diarylmethanes from a Series of Analogous Quantum Chemical Models.
Finding simple effective Hamiltonians for classes of complex chemical systems is not easy. Justifying them from quantum chemistry is even harder. We consider a family of organic dye molecules related to Michler's hydrol blue, including auramine-O and malachite green. These molecules are increasingly being used as sensors of the local environment in biomolecules.
The three lowest singlet states can be described by a 3x3 matrix Hamiltonian whose parameters vary across the family of the dyes. This variation appears to be correlated with an empirical parameter [Brown-Okamoto] used to characterise the effect of substituents. There is also a subtle and interesting variation in the character of the diabatic states as one traverses the family of dyes.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



Hi Ross,
ReplyDeleteNice paper :). I have a few comments, if you like.
In 2.2, when introducing the Hamiltonian, I think the point that this is the most general 3 state model allowed by the symmetry could be made earlier (and stronger), before writing any of the individual elements.
The identification of |L>, |R>, |B> as left, right and bridge states could be clearer; possibly even inspired by symmetry arguments when introducing H.
I think the labeling in Fig 2 might be wrong; should the purple "l2b2" state be "b2r2"?
Thanks Jacko; I'm glad you enjoyed it. You're right about the figure error - thanks again, and I'll give the paragraph in 2.2 some special attention. Hope all's well over yonder.
ReplyDelete