How important are electron-electron interactions in organic molecules?
There is a really nice old (1975) paper Why is azulene blue and anthracene white? by Michl and Thulstrup, which highlights the relevant issues.
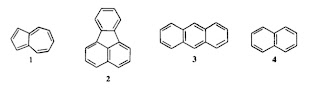
They point out that these two molecules 1 and 3 have similar ionisation energies (IP) and electron afinities (EA) but their lowest lying singlet (S) and triplet (T) excited states have quite different energies (see the Table below). If a simple molecular orbital (Huckel) picture which ignores electron-electron interactions was valid than the energy difference between the LUMO and HOMO would equal IP-EA = T = S.
However, the Table clearly shows this is not the case.
At the Hartree-Fock level the discrepancies provide a measure of
J = Coulomb integral ~ Hubbard U ~ 5 eV
2K= Exchange integral = Singlet-Triplet splitting ~ 1 eV.
Michl and Thulstrup give a nice simple explanation of why the exchange integral K is smaller in azulene than in anthracene. (This is what leads to the different colours). Azulene is a non-alternating hydrocarbon (5 ring + 7 ring) and so the spatial overlap of the HUMO and LUMO is much smaller than for anthracene.
The fact that Hartree-Fock captures some of the above does not mean that electron correlations are not important. I think the fact that J is comparable to the energy splitting of the HOMO and LUMO estimated from IP and EA suggests configuration interaction in the singlet excited states may be significant.
p.s. There is an old post Am I LUMO-phobic? plus comments which discusses the issues. A recent paper by Paul Schwenn, Paul, Burn, and Ben Powell showed that some of the claimed "success" at DFT based methods calculating IP and EA is due to a fortuitous cancellation of errors.
Subscribe to:
Post Comments (Atom)
Maxwell's demon and the history of the second law of thermodynamics
I recently reread Warmth Disperses and Time Passes: The History of Heat by Hans Christian von Baeyer As a popular book, it provides a beaut...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...




No comments:
Post a Comment