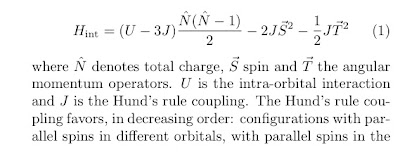
Writing down the simplest possible effective Hamiltonian for a chemically and structurally complex material is a great challenge. This is particularly true of transition metal compounds because of the presence of multiple d-orbitals. Often crystal field effects split these orbitals and so one needs only consider a subset. Nevertheless, justify a selection and defining the relevant inter and intra-orbital interactions is difficult. Starting from the atomic limit one can consider the following Hamiltonian for a single atom [see this post about Hund's rule]
same orbital, and with opposite spins in the same orbital, maximizing S and then T.
This seems a lot simpler and transparent than forms I have seen with creation and annihilation operators.
How does one determine U and J? Hotta has a helpful review where he discusses these in terms of Racah (Slater) parameters. He also discusses the overlap matrix elements associated with orbitals on neighbouring atoms. These are needed to determine a tight-binding band structure.
One should also be able to extract some of these parameters from related transition metal complexes studied by inorganic chemists.




No comments:
Post a Comment