- Am I HOMO- and LUMO-phobic? (476)
- Mind the gap (230)
- Spectral density of water (162)
- 20 key concepts in thermodynamics and condensed matter physics (132)
- Can we see visons? (107)
- How can living organisms be so highly ordered? (101)
- Walter Kauzmann: master of thermodynamics (98)
- James Bond meets Niels Bohr (96)
- Electron versus hole transport in molecular materials (92)
- Quantum coherence in photosynthesis (91)
Thursday, December 31, 2009
Most viewed posts
Wednesday, December 30, 2009
Emergence in economics
Paul Krugman was awarded the Nobel Prize in Economics in 2008. He is also a New York Times columnist. Why mention him here? He is mentioned in Steven Johnson's book Emergence (p. 89-91) for a mathematical model which describes how spatially segregated business centres emerge. This is described in Krugman's book, The Self-Organising Economy.
Here are Krugman's "rules for research", taken from a talk, "How I work"
1. Listen to the Gentiles
2. Question the question
3. Dare to be silly
4. Simplify, simplify
1. means "Pay attention to what intelligent people are saying, even if they do not have your customs or speak your analytical language."
Emergence is universal
- More is different
- Ignorance is useful
- Encourage random encounters
- Look for patterns in the signs
- Pay attention to your neighbours
Monday, December 28, 2009
I am in more than one mind about this...
- The Disciplinary Mind: You need to master a specific discipline or research area. This takes about ten years.
- The Synthesizing Mind: You need to learn to integrate ideas from different disciplines into a coherent whole and to communicate that integration to others.
- The Creating Mind: You need to develop the capacity to uncover and clarify new problems, questions and phenomena.
- The Respectful Mind: You need to be aware of and appreciate different approaches and values within your discipline and between disciplines.
- The Ethical Mind: You need to fulfill your responsibilities as a worker within your institution, your discipline, and as a citizen.
Thursday, December 24, 2009
Embrace failure?

Seth Olsen alerted me to a thought-provoking article in Wired about the importance of failure in science.
Wednesday, December 23, 2009
Think before you calculate, measure, fabricate,....
Monday, December 21, 2009
Electronically driven hula dancers
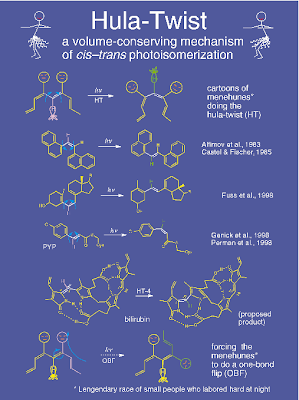
Saturday, December 19, 2009
Trust, but verify

Thursday, December 17, 2009
Talks that go pear shaped....
Wednesday, December 16, 2009
How big a Hilbert space do you need?


Sunday, December 13, 2009
When business people think they can run a scientific organisation...
Saturday, December 12, 2009
Listening to referees
Thursday, December 10, 2009
Networking with Nobel laureates

My family continues to enjoy watching the TV show The Big Bang Theory. In the episode we watched tonight Sheldon unsuccessfully tried to give Nobel Laureate George Smoot (appearing as himself) a copy of his latest paper at a conference.
Wednesday, December 9, 2009
When does the wavefunction collapse in nuclear collisions?
Tuesday, December 8, 2009
Friction in nuclear collisions
Monday, December 7, 2009
In the wrong place?
Decoherence and irreversibility


Sunday, December 6, 2009
Mental health issues for researchers

Saturday, December 5, 2009
Quantum limited detectors
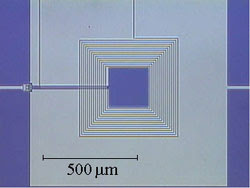
Friday, December 4, 2009
Images of condensed matter physics
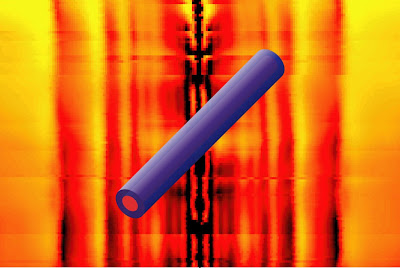
Deconstructing charge transport in complex materials II
Thursday, December 3, 2009
Read the ad, answer the ad
Wednesday, December 2, 2009
Model Hamiltonian parameters from electronic structure theory
Tuesday, December 1, 2009
Monday, November 30, 2009
What are deconfined spinons?


Sunday, November 29, 2009
THE question
What does the scientific community know now that we did not know when you began the research?There is a similar probing question to ask yourself before you start a project (or a new sub-project). Suppose everything goes as well as can be hoped (i.e., you are able to complete the calculation, do the measurement, get the new technique to work, or make the compound). Then will you be able to say something new? If not, is it worth even trying?
The before question is a good one for both students and supervisors to contemplate. It is too easy for supervisors (including me) to say do this extra calculation (or make this extra compound and measure all its properties) without considering enough the time cost to the student or postdoc.
Thursday, November 26, 2009
The most important letter in your scientific career?
The cover letter is key.
I believe most postdoc (and many faculty) applications live or die [i.e., get to the long short list] based on the quality of the cover letter.
You need to specifically answer the following specific questions:
- Why are you interested in this specific job?
- Why are you interested in this specific research?
- Why should they hire specifically you for this specific job?
So to be specific!
Write something like:
"One of the scientific questions I am most interested in is "What is the physical mechanism for XXXX in material YYY? I recently read your nice paper "blah blah" in journal YY and I have been wondering if a similar approach might be relevant to answering my question. I welcome any suggestions from you in this regard....."Dont write:
"Dear Professor,The letter is so important you should spend at least a day writing it, even though is should fit on a single page. Should should get a range of faculty to read it and provide feeback.
I did my Ph.D on topic X and want to continue working on it [even though I have no idea as to whether you have ever worked in this area or have any interest in it]. I published lots of papers [even though most listed on the CV are "in preparation"] and will publish lots more if I come and work with you....]"
Wednesday, November 25, 2009
Diverse career options for physicists
 Here are the slides of the fascinating talk that Joel Gilmore gave at the Careers session organised by the Australian Institute of Physics (Qld branch) last wednesday.
Here are the slides of the fascinating talk that Joel Gilmore gave at the Careers session organised by the Australian Institute of Physics (Qld branch) last wednesday.
Deconstructing charge transport in complex materials
The chemical capacitance has certain similarities to conventional dielectric capacitance. While the latter is a measure of the ability of the system to store electrical energy in the form of polarized electric dipoles, the former is a measure of the ability of the system to store chemical energy in the form of changes in stoichiometry

Tuesday, November 24, 2009
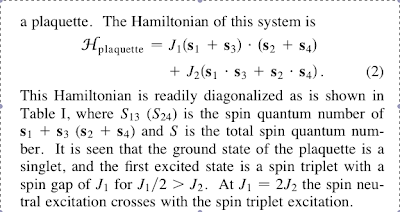
Quantum frustration in a nutshell

Sunday, November 22, 2009
Tough times for science in California
Saturday, November 21, 2009
Teaching high school physics
Richard is also co-author of a high school physics textbook,

that Joel Gilmore (Science communicator extraordinaire and my former Ph.D student) was so impressed with he bought it off a student he was tutoring when she finished high school!
Friday, November 20, 2009
Our tendency to scientific fantasy not reality
“The great power of science is its ability, through brutal objectivity, to reveal to us truth we did not anticipate.”
(p. xvi)
``mythologies are immensely powerful things, and sometimes we humans go to enormous lengths to see the world as we think it should be, even when the evidence says we are mistaken.’’
(p. 114)
“ideologies preclude discovery. All of us see the world as we wish it were rather than as it actually is.”
(p. 116).
There are similarities to the cautions of Walter Kauzmann, in his Reminiscences of a Life in Protein Chemistry.
Thursday, November 19, 2009
Careering out of control

Here is a copy of the talk I gave tonight on academic careers.
Some of the feedback included:
* the importance of mental health issues (I will try and do a few future posts on this).
* dogged perseverance is often a key component to success
It would be good to get some discussion going on some of the issues I raise in the talk.
Are your perceptions of stress objectively accurate?
Kreger DW. Wright Institute, Berkeley, CA 94704, USA.
Psychological Reports 1995 Feb;76(1):345-6.
In a study of 29 graduate students, self-ratings of stress correlated with low scores on self-esteem but were not related to an objective indicator of actual stress. Both self-rated stress and low self-esteem scores were related to scores on depression, with a weak interaction effect.
Wednesday, November 18, 2009
Advice on a research career
There is more to life than a research career.
Be realistic and consider alternative careers.
Learn to write, to get along with other people, ....
plus previous career advice I have posted, especially this advice to Ph.D students.
When was the first BEC observed?
But it should be noted that the case of a BEC in superfluid He is not as clear cut as in dilute atomic gases. Nevertheless, I dont think these subtleties validate ignoring 80 years of research on superfluid helium. A very useful summary of the history and the associated physical issues is contained in this nice article by Sebastian Balibar.
I think that people who are supervising Ph.D students on BEC's should be familiar with these issues, make sure their students are aware of this history, and present their work in the appropriate context. But then I am just a grumpy old condensed matter physicist....
Tuesday, November 17, 2009
Organic LED's in nature
 Just how efficient are biomolecular systems? For a long time it was claimed that fireflies had a quantum efficiency close to 100 per cent. However, it was recently found this is not the case, the efficiency being about 40%. A nice summary of the work is in this News and Views article in Nature Photonics last year.
Just how efficient are biomolecular systems? For a long time it was claimed that fireflies had a quantum efficiency close to 100 per cent. However, it was recently found this is not the case, the efficiency being about 40%. A nice summary of the work is in this News and Views article in Nature Photonics last year.Besides measuring the quantum efficiency Ando et al. find that there are three components to the light emission and all are pH dependent. One component is of unknown origin. Clearly there is a correlation between the colour of the emission and the protonation state of the chromophore.
Only in a 2006 Nature paper was a structural basis for two different emission states proposed. I am curious as to how much quantum chemistry has been done on these issues. This may help address questions such as:
What determines the quantum efficiency of emission?
How are non-radiative decay channels suppressed?
What role does the protein environment play?

Monday, November 16, 2009
What is your goal?
What is the scientific question you want to answer in the next few years?
Why is this important? Why are you excited about it?
Where is Brisbane anyway?
They really put the size of Australia in perspective.


Saturday, November 14, 2009
Frustrated quantum spin models in a nutshell
The simplest of these problems involve only the spin operators Si of electrons residing on the sites, i, of a regular lattice. Each electron can have its spin oriented either up or down, leading to a Hilbert space of 2N states, on a lattice of N sites. On this space acts the Heisenberg Hamiltonian
H=∑i<j JijSi⋅Sj, (1)where the Jij are a set of short-range exchange interactions, the strongest of which have Jij>0, i.e., are antiferromagnetic. We would like to map the ground-state phase diagram of H as a function of the Jij for a variety of lattices in the limit of N→∞. Note that we are not interested in obtaining the exact wave function of the ground state: this is a hopeless task in dimensions greater than one. Rather, we would be satisfied in a qualitative characterization of each phase in the space of the Jij. Among the possible phases are
(i) a Néel phase, in which the spins have a definite orientation just as in the classical antiferromagnet, with the spin expectation values
all parallel or antiparallel to each other; (ii) a spiral antiferromagnet, which is magnetically ordered like the Néel phase, but the
spins are not collinear; (iii) a valence bond solid (VBS), with the spins paired into S=0 valence bonds, which then crystallize into a preferred arrangement that breaks the lattice symmetry; and
(iv) a spin liquid, with no broken symmetries, neutral S=1/2 elementary excitations, and varieties of a subtle “topological” order.
Specific examples occur for Heisenberg models on the (i) square lattice, (ii), triangular lattice, (iii) kagome lattice (probably).
An example of a model which contains all three is here.
We are still searching for an example of (iv).
Friday, November 13, 2009
Twisting charges apart
An important issue is after a organic molecule absorbs a photon what conformational change will occur. Below are several options involving bond twists.
 A second issue is how the charge distribution in the molecule changes upon twisting.
A second issue is how the charge distribution in the molecule changes upon twisting.This kind of physics is at the heart of how your eye works. When retinal absorbs a photon it undergoes a conformational change which produces a charge separation which eventually leads to an electrical signal in your brain. It is also at the heart of designing better organic solar cells.
We considered a model Hamiltonian for a large class of dyes. The figure below shows contour plots for the first excited state potential energy surface for several parameter values. The lower part of the figure shows how the charge distribution in the molecule changes for different conformations. It is amazing how such a simple model Hamiltonian can capture such rich physics.

Thursday, November 12, 2009
Wednesday, November 11, 2009
Computational modeling of complex chemical systems: the state of the art
This is a question that Donald Truhlar asks in a JACS Editorial for a Select issue of 23 papers on Molecular Modeling of Complex Chemical Systems.
How would you answer the question?
You can look in the article to see how most computational chemists would answer the question.
The article is a very nice read to a physicist because it provides a very helpful and concise summary of historical landmarks in the computational modeling of large chemical systems.
However, I disagree and am concerned with one of the opening statements in the article:
Almost all modern theoretical chemistry is computational chemistry, because most of the progress that can be made with pencil and paper without a computer has been already made. Computations on complex systems are, in my opinion, the current frontier of theoretical chemistry.I fear this does reflect the view of most in the theoretical chemistry community. However, (as a physicist) I think a much greater emphasis needs to be placed on gaining insights, finding organisation principles, and developing analytical models that complement simulations. But, that is what I am trying to do (and excited about!).
Tuesday, November 10, 2009
Many worlds or many words?
“purely a matter of taste, roughly equivalent to whether or not one believes mathematical language or human language to be more fundamental.”But it is interesting that he is now writing articles about multiverses...
Monday, November 9, 2009
Schrodinger was right on the money!
The decay path taken
A key question concerning optically active molecules is what is dynamics of the excited states?
Specifically, what are the predominant non-radiative decay mechanisms. The schematic below shows several options for the energy of the potential energy surfaces versus some configurational co-ordinate. On the left both S1 and S2 excited states decay to a conical intersection with the ground state. In contrast, on the right they have distinctly different decay paths.

But how does one go beyond such schematics. It turns out that for a broad class of dyes one can justify from high level quantum chemistry calculations a description in terms of just three valence bond states (see below).
 The description in terms of the three diabatic states allow us to consider a somewhat "generic" or minimal model which exhibits a number of significant features, including:
The description in terms of the three diabatic states allow us to consider a somewhat "generic" or minimal model which exhibits a number of significant features, including:- Conical intersections between the S1 and S0 surfaces only occur for large twist angles.
- In contrast, S2/S1 intersections can occur near the Franck-Condon region.
- When the molecule has left-right symmetry, all intersections are associated with con- or dis-rotations and never with single bond twists.
- For asymmetric molecules (i.e. where the bridge couples more strongly to one end) then the S2 and S1 surfaces bias torsion about different bonds.
- Charge localization and torsion pathway biasing are correlated.
BTW, if you look at the paper look at the great job Seth did at writing Informative section headings!
Sunday, November 8, 2009
MO vs. VB for ketone dyes
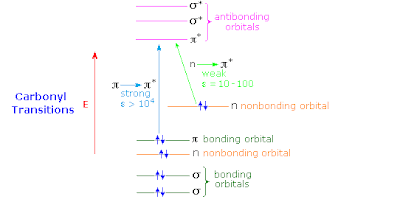

Thinking in the alternative resonating valence bond picture there will be three alternative Lewis structures
O
||
C -R
|
L
O-
|
C -R
|
L+
O-
|
C -R+
|
L
The extent to which the lower two structures contribute will increase the C-O bond length and reduce the C-O stretch frequency.
It should be possible to describe the low lying excited states in terms of a complete active space with 4 electrons in 3 orbitals (a pi* orbital on the C=O bridge, and a pi orbital on the left and right fragments).
Saturday, November 7, 2009
Inventing your mother-in-law or More is Different II
Anderson suggests that emergence is the mechanism for consilience (the unifying of disparate pieces of knowledge) and reduction is the evidence for it. Theories may be under-determined, i.e., there may be many possible theories that can explain what is actually known. Hence, a successful theory may not actually correspond to what is happening. If there are only a few constraints (hypotheses, observations) that a theory must satisfy it has sometimes been the case that more than one theory can satisfy the constraints. However, as the number of constraints increases, acceptance of a theory is more likely and it becomes hard to conceive of alternative theories that could satisfy these constraints. Reduction can greatly increase the number of conditions that a theory must satisfy. For example, any alternative to quantum theory must be able to explain all known principles of atomic physics and of chemistry. Anderson concludes ``it is as impossible to `socially construct’ science as it is to invent A. Abrikosov or your mother-in-law.’’
Anderson mentions work of Kirkpatrick concerning the problem of satisfying many constraints. An example is this Science paper.
Friday, November 6, 2009
Pauling and Bardeen on postage stamps

It is wonderful that last year the US Postal Service issued new stamps featuring four prominent scientists. Pauling and Bardeen were indeed masters in unravelling emergent phenomenon using quantum many-body theory.
Update (2016). I just discovered that in 2005 there was one for Josiah Willard Gibbs and Feynman.
Thursday, November 5, 2009
Modelling a class of organic dyes
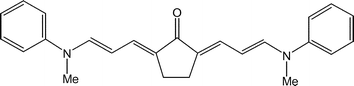
Ketocyanine dyes are of considerable interest. An example is shown above. What are the essential ingredients that determine their photophysical properties? The figure below is from a nice paper which compares essential differences between cyanines (CY), ketocyanines (KCY), and squarenes (SQ).

The difference between the upper and lower panels (A and B) relates [I think] to whether one has an even (A) or odd (B) number of p-electron centres on each of the molecular units on the left and right side of the central C=O bridge. Apparently, this is following a "composite molecule" approach in a book by Fabian and Hartmann.
A complementary approach to describing optical properties of these materials is within a resonating valence bond approach. There will be three dominant resonant structures, similar to those advocated by Pauling for urea. [I will try and get a picture]. Such an approach will naturally lead to two low-lying singlet excited states.
This type of resonance is of great biochemical significance since it leads to the planarity of amide groups in polypeptides, something figured out by Pauling and Corey in 1952, an emphasized because it is required for the alpha helix of proteins.

A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...






