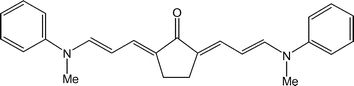
Ketocyanine dyes are of considerable interest. An example is shown above. What are the essential ingredients that determine their photophysical properties? The figure below is from a nice paper which compares essential differences between cyanines (CY), ketocyanines (KCY), and squarenes (SQ).

The difference between the upper and lower panels (A and B) relates [I think] to whether one has an even (A) or odd (B) number of p-electron centres on each of the molecular units on the left and right side of the central C=O bridge. Apparently, this is following a "composite molecule" approach in a book by Fabian and Hartmann.
A complementary approach to describing optical properties of these materials is within a resonating valence bond approach. There will be three dominant resonant structures, similar to those advocated by Pauling for urea. [I will try and get a picture]. Such an approach will naturally lead to two low-lying singlet excited states.
This type of resonance is of great biochemical significance since it leads to the planarity of amide groups in polypeptides, something figured out by Pauling and Corey in 1952, an emphasized because it is required for the alpha helix of proteins.





No comments:
Post a Comment