Just like for other systems such as superconductors their interpretation is subtle.
The first studies are reviewed in Section 2.3.5 of this review article.
The presence of hysteresis and the difference between the transition temperature for
increasing and decreasing
temperature sweeps are determined by the magnitude of the Ising interactions.
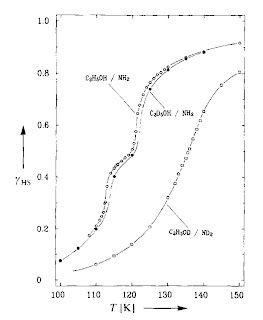
Isotopic exchange was investigated for a tris(picolylamine)iron(II) system which exhibits a two-step spin transition. Results are shown in the figure below. Significant changes in the spin-state transition curve were observed only when the isotopic substitution
(H/D and 14N/15N) was made for atoms directly involved in the hydrogen-bonding network that connects the spin-crossover molecules.
For example, with C2H5OD/ND2 the crossover temperature was shifted to higher temperatures by about 15 K and the middle step was no longer present.
I would not have expected such a large effect given the chemical complexity of these systems and that the H atoms are not immediately bonded to the iron atoms which undergo the spin-state transition.
I now mention two other studies. They are particularly helpful because they also measured how the enthalpy and entropy change associated with the spin-state transtion changed with isotopic substitution.
Weber et al. studied the
iron(II) spin-crossover complex [FeL1(HIm)2] and the deuterium-substituted [FeL1(DIm)2] where Him is (not a man but) imidazole. Both exhibit a single-step transition
with hysteresis. H/D exchange decreased both the transition temperature and the hysteresis width by a few K. Deuteration decreased the value of the enthalpy and entropy differences between the low spin and high spin states (determined from
differential scanning calorimetry) by about twenty and ten percent, respectively. (See Table 2 in the paper). They estimated an interaction parameter J = 560 K, indicating strong intermolecular interactions, which they attributed to a hydrogen bond.
They reference some earlier studies showing how the magnitude of the ligand field in a transition metal complex can be modified by hydrogen bonds involving the complex.
Very recently, Jornet-Mollá et al. studied the iron(ii) salt [Fe(bpp)2](isonicNO)2·HisonicNO·5H2O, which with decreasing temperature undergoes a transition at 162 K. There is a width of about 5 K, associated with hysteresis.
With deuteration, the transition temperature decreases to 155 K, the width increases to 7 K, and the enthalpy and
entropy differences both increase by about fifteen percent.
“Annealing the compound at lower temperatures results in a 100% LS phase that differs from the initial HS phase in the formation of a hydrogen bond (HB) between two water molecules (O4W and O5W) of crystallisation. Neutron crystallography experiments have also evidenced a proton displacement inside a short strong hydrogen bond (SSHB) between two isonicNO anions.”
I am particularly interested in this because of previous work I have done on strong hydrogen bonds.
Again I am surprised at the magnitude of these effects because the zero-point energy associated with the relevant H atoms is only a small fraction of the total zero-point energy and the entropy contribution from vibrations.
I now start a preliminary discussion of how these experiments might be interpreted in terms of an Ising model picture, such as in a recent preprint. The Hamiltonian is
The crossover temperature is independent of the Ising interactions J's and given by
Thus, it is a property of the individual molecules and not directly dependent on the intermolecular interactions.
Our results in Appendix A of the preprint imply that there should be no dynamical isotope effects on the J’s, i.e., provided other parameters such as structural details and bond lengths do not change with isotope substitution.
This does not rule out
changes in the crossover temperature. Both the enthalpy and entropy differences can change with isotope substitution (as is observed). The former due
to changes in zero-point energies, and the latter due to changes in the vibrational contribution to the entropy change.






Very interesting work- I wonder how much the intrinsic sample variability is for these materials though? One can often freeze in all sorts of disorder etc in molecular based materials. One of those boring, but just possibly important things that I hope people check!
ReplyDelete(just to be clear, the above is related to the apparent change in T_sc upon deuteration)
ReplyDelete