Carbonic anhydrase is a common enzyme that performs many different physiological functions including maintaining acid-base equilibria. It is one of the fastest enzymes known and its rate is actually limited not by the chemical reaction at the active site but by diffusion of the reactants and products to the active site.
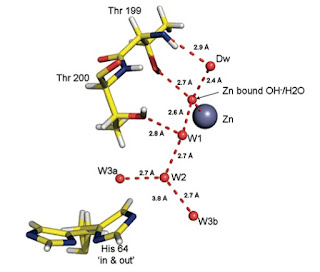
Understanding the details of its mechanism presents several challenges, both experimentally and theoretically. A key issue is the number and exact location of the water molecules near the active site. The most recent picture (from a 2010 x-ray crystallography study) is shown below.
The "water wire" is involved in the proton transfer from the zinc cation to the Histidine residue. Of particular note is the short hydrogen bond (2.4 Angstroms) between the OH- group and a neighbouring water molecule.
Such a water network near an active site is similar to what occurs in the green fluorescent protein and KSI.
Reliable knowledge of the finer details of this water network really does matter.
This ties in with theoretical challenges that are related to several issues I have blogged about before. Basic questions concerning proton transport along the wire include:
A. Is the proton transfer sequential or concerted?
B. Is quantum tunnelling involved?
C. What role (if any) does the dynamics of the surrounding protein play?
A 2003 paper by Cui and Karplus considers A., highlighting the sensitivity to the details of the water wire.
Another 2003 paper by Smedarchina, Siebrand, Fernández-Ramos, and Cui looks at the both questions through kinetic isotope effects and suggests tunnelling plays a role.
In 2003 it was not even clear how many water molecules were in the wire and so the authors considered different alternatives.
One can only answer these questions definitively if one has extremely accurate potential energy surfaces. This is challenging because:
Barrier heights and quantum nuclear effects vary significantly with small changes (even 0.05 Angstroms) in H-bond donor-acceptor distances.
The potential surface can vary significantly depending on the level of quantum chemistry theory or density functional that is used in calculations.
I thank Srabani Taraphder for introducing me to this enzyme. She has recently investigated question C.
Subscribe to:
Post Comments (Atom)
A forgotten physicist: Amelia Frank (1906-1937)
In honour of International Women's Day, I bring to your attention a fascinating recent piece in The Conversation , Who was Amelia Frank?...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



My comment is not about the current post, but is about the Anderson paper that appear today,14/12/2016, in the Arxiv called "LAST WORDS ON THE CUPRATES". This paper deserve
ReplyDeletea particular post in your beautiful blog, I think.
The link is https://arxiv.org/pdf/1612.03919.pdf
ReplyDelete