I would be interested to hear feedback on the claim.
Consider some chemical reaction
A + B to C + D
One can consider the reaction is the gas phase [i.e. without an environment] or in a solvent [polar or non-polar] or in a protein environment. The relative stability of the reactants and the products, the rate of the reaction, and the reaction mechanism [i.e. the reaction co-ordinate and transition state geometry] can vary significantly and dramatically.
This is what is amazing about enzymes: you can increase the rate of a reaction by a factor of a billion.
So what is the most basic hypothesis about the effect of the environment? It can do two significant things.
1. The bond lengths of A and/or B and/or their relative geometry is changed by the environment. For example, A and B are forced closer together.
2. A polar environment [e.g. water or a protein] can change the relative energies of the transition state, and/or the reactants and products. This is highly likely because most molecules have non-uniform charge distributions and significant dipole moments.
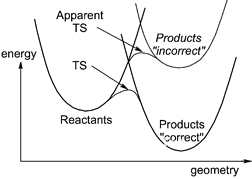
This claim has a natural understanding in terms of a simple diabatic state picture for the reaction. The environment can change the shapes of the diabatic potential energy surfaces and/or change the strength of the coupling of the two surfaces. [For example, in the figure below replace "incorrect" with "no environment" and "correct" with "environment"].
Why is this claim boring?
Well it means that there is nothing that "special" or unique about proteins. There is no new physics or chemistry.
It rules out exotic mechanisms such as dynamic effects and particularly collective quantum effects.
Finding out how an environment does change specific parameters is highly non-trivial.
Furthermore, outstanding, fascinating, and difficult problems remain understanding and describing:
- how the protein "engineers" the changes in the reaction potential energy surface,
- how mutations distant from the "reaction centre" can sometimes have such a significant effect,
- the role of the hundreds of amino acids not close to the "reaction centre", i.e. why do proteins need be so big? is there a lot of redundancy? or does one really need all those amino acids to produce a highly "tuned" and exquisite tertiary structure?
So is this claim "controversial" or "dogma" or "obvious"?




There are 50 or more years of experiment and theory in biophysics and biochemistry related to your claim. I am an enzymologist and have read a lot of this work. I have done some of it.
ReplyDeleteYou claim is not obvious. It is not controversial, exactly. Most people do not think about how enzymes speed up reaction rates.
A simple answer is that enzymes trap reaction intermediates that are higher energy than the normal state of a molecule and hold these intermediates in the reactive configuration until a reaction takes place. Enzymes enrich the solution for active intermediates.
There is a much longer version of the above statements in terms of quantum mechanics, amino acid sequences, statistical mechanics, and genetics. Ask for the longer version if you want it.