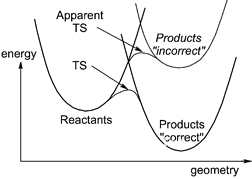
A typical group consists of one to a few faculty members and postdocs, grad. students, and possibly some undergrads working in the group.
It is important that these weekly meetings are informal, relaxed and inclusive.
They should encourage learning, interaction and feedback.
What might happen at the meeting?
Here are a few things that we do in the Condensed Matter Theory group at UQ [senior faculty are Ben Powell and I].
These meetings are compulsory and they are in addition to a weekly meeting between each individual in the group and their supervisor.
Each week a group member is assigned to bring a cake or a packet of cookies/biscuits to share with the group. Group members provide their own drinks.
- A group member gives a talk on the white board about something they are currently working on or a tutorial on a specific subject. Questions, particularly basic ones from students, are encouraged during the talk. Powerpoint is only allowed when essential for graphs of results.
- A group member gives a practise talk for an upcoming speaking engagement, whether for a seminar, conference, or a Ph.D progress report. Detailed feedback is given, both about scientific content and presentation, afterwards.
- Everyone in the group brings a paper they recently read and each has 7 minutes to convince the audience they should also read the paper.
- Journal club. Everyone reads a pre-assigned paper and it is discussed in detail.
Once for a few months we ran a competition each week to see who could ask the most questions during the talk. The winner got a bottle of wine; but had to provide the prize for the following week.
Some large groups do need to have regular meetings to discuss issues such as lab maintenance, software development, .... I think it is important that these mundane but important issues not crowd out science and so they should be held separately or fenced off to a second hour.
We have also had more specialised sub-group meetings that run for a limited time such as the following.
- Reading groups. Working through a specific book, one chapter per week, e.g. those by Fulde, Phillips, and Hewson.
- Student only meetings. This gives them a greater freedom to teach each other and to ask basic questions.
- Joint theory-experiment or chemistry-physics meetings, usually focused on a specific collaboration.
Our meetings are far from perfect. But I think the most important thing is to keep meeting and experiment with different formats.
What is your experience?
What do you think has contributed to the most effective and enjoyable meetings?
What do you think has contributed to the most effective and enjoyable meetings?