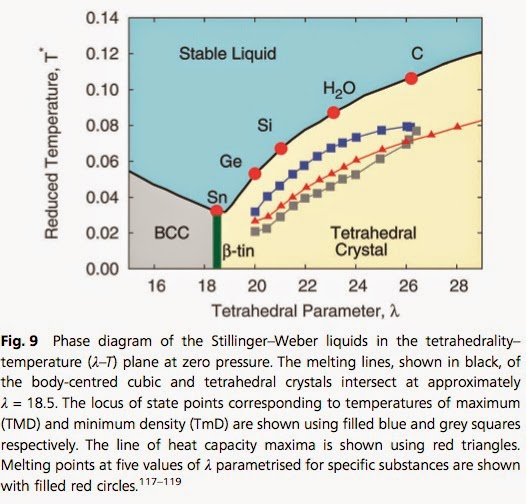
At the NORDITA water meeting Charusita Chakravarty gave a nice talk that featured the phase diagram below.
The figure is taken from a nice Perspective paper in PhysChemChemPhys.
Water and water-like liquids: relationships between structure, entropy and mobility
Divya Nayara and Charusita Chakravarty
The article gives a nice overview, putting the anomalous properties of water in a broad context, comparing and contrasting to the properties of other liquids for which tetrahedral interactions are dominant. Possible relations between thermodynamics, transport, and structure are also discussed.
Key anomalous properties of water [compared to simple isotropic liquids] include
-the negative slope of the melting line in the temperature-pressure phase diagram
-the temperature of maximum density [277.15 K at 1 atm]
-increase in diffusion with increasing density
-increase in specific heat, thermal expansion, compressibility upon isobaric supercooling.
Water is actually not as unique as I thought. Other tetrahedral liquids exhibit similar anomalies. Furthermore, it is not the hydrogen bonding (per se) that makes water anomalous, but rather the tetrahedral interactions associated with the hydrogen bonding.
The figure above is based on the Stillinger-Weber model, a coarse-grained model that captures the competition between two-body interactions and three-body (tetrahedral) interactions. The version of the model for water is termed monatomic Water (mW) and is described in this previous post. At the meeting Jibao Lu described recent work which gave an objective scoring of the successes and failures of different mW models and atomistic models.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



I think I understand why tetrahedral structure implies 3-body interactions, but can I verify: the sp3 structure of water (and other tetrahedral atoms) is due to VESPR interactions i.e. the formation of pairs and inter-pair repulsion, which is a 2-body effect. The interaction of a sp3 orbital with another water would involve the interaction of the pair on the "atom" with the particles on another atom. Hence, 3-body. Is this essentially right?
ReplyDelete