I really enjoyed this week's meeting
Water: the most anomalous liquid at NORDITA. This is the first time I have ever been to a workshop or conference that is solely about water. Here are some impressions and a few things I learnt as a newcomer to the field.
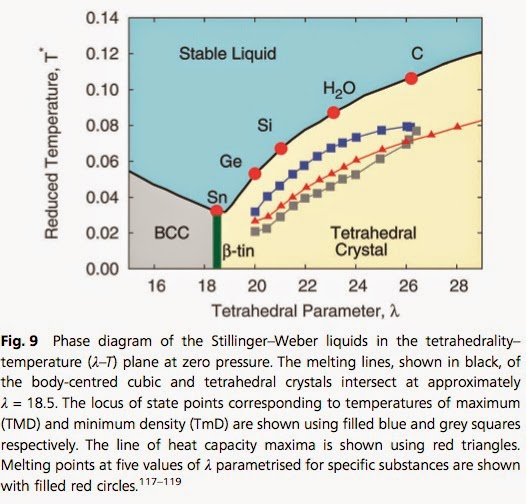
Just how unique and anomalous is water?
Not as unique as I thought. Some other tetrahedral liquids have similar properties.
Hydrogen bonding is not what makes water unique
Rather it is the tetrahedral character of the intermolecular interactions that arise from hydrogen bonding. This distinction can be seen from the fact that the
mW (monatomic water) model captures many of the unusual properties of water.
DFT is a nightmare
I have written a number of posts that express caution/concern/alarm/skepticism about attempts to use Density functional theory (DFT) to describe properties of complex materials. Trying to use it to calculate properties of a liquid water in thermal equilibrium is particularly adventurous/ambitious/reckless. First, there is the basic question: can it even get the properties of a water dimer in the gas phase correct? But, even if you choose a functional and basis set so you get something reasonable for a dimer there is another level of complexity/fakery/danger associated with "converging" a molecular dynamics simulation with DFT producing the Born-Oppenheimer surface. This was highlighted by several speakers. Simulations need to give error bars!
A physically realistic force field (at last!)
A plethora of force fields [TIP3P, SPC/E, TIP4P/2005, ST2, ....] have been developed for classical molecular dynamic simulations. They are largely based on electrostatic considerations and involve many parameters. The latter are chosen in order to best fit a selection of experimental properties [melting temperature, temperature of maximum density, pair correlation function, dielectric constant, ....]. Some models use different force fields for ice and liquid water. On the positive side it is impressive how some of these models can capture qualitative features of the phase diagram including different ice phases and give a number of experimental properties within a factor of two. On the negative side: they involve many parameters, it is hard to justify including some "forces" and not others, and give very poor values for some experimental observables [e.g. TIP3P has ice melting at 146 K!]. How often do people get the right answer for the wrong reason?
An alternative strategy is to actually calculate an
ab initio force field using state of the art quantum chemistry and a many-body expansion that includes not just two-body interactions (i.e. forces between pairs of molecules) but three-body and beyond interactions. This was discussed by
Sotiris Xantheas and
Francesco Paesani. An end result is
MB-pol.
Quantum zero-point energy is (not) important
Sotiris Xantheas emphasised that semi-empirical force fields are effective Hamiltonians that implicitly include quantum nuclear effects at some effective classical potential [e.g.
a la Feynman-Hibbs]. Thus, if one then does a path integral simulation using one of these force fields one is "double counting" the quantum nuclear effects at some level. Xantheas and Paesani also emphasised that MB-pol should not be expected to agree with experiment unless nuclear quantum effects are included.
On the other hand, due to competing quantum effects classical simulations for water give better results than one might expect.
The elusive liquid-liquid critical point
Some of
this controversy reminded me of high-Tc cuprate superconductors where the elusive quantum critical point [
under the superconducting dome?] may (or may not) exist. It is also interesting that there is a
proposal of a Widom line in the cuprates, perhaps inspired by water.
Some of the arguments and sociology seemed like the cuprates. There are true believers and non-believers. Each camp interprets (and criticises) complicated and ambiguous experimental results and large computer simulations according to their prior beliefs. Kauzmann's maxim
is relevant: people will often believe what they want to believe rather than what the evidence before them suggests they should believe.
Perhaps this critical point does not appear in the physical phase diagram of bulk water but can be accessed via "negative pressure" in some force field models. A key observable to calculate is the heat capacity, experimentally it appears to diverge. But its calculation will require inclusion of nuclear quantum effects. [It is not clear to me why you can't just input the classical vibrational spectrum into a non-interacting quantum partition function.]
I felt this issue dominated some discussions at the meeting too much.
The O-O radial distribution function is over-emphasised
In any liquid this
pair correlation function is an important observable that is a measure of the amount of structure in the liquid. For water the O-O radial function has been "accurately" measured and provides a benchmark for theories. Getting it correct is a necessary
but not a sufficient condition for having a correct theory. But water is an anisotropic molecular liquid not a Lennard-Jones monatomic fluid. Angular correlations are very important for water. Also, unfortunately, other pair correlation functions such as the O-H and H-H radial distribution functions are not well characterised experimentally.
When are the many-body effects quantum?
One can make many-body expansions in electrostatics, classical statistical mechanics, and quantum many-body theory. A profound question is: are there situations, criteria, or properties that can make the latter distinctly different from the former?