There is a nice helpful review
Biochemistry and Theory of Proton-Coupled Electron Transfer
Agostino Migliore, Nicholas F. Polizzi, Michael J. Therien, and David N. Beratan
Here are a few of the (basic) things I got out of reading it (albeit on a long plane flight a while ago).
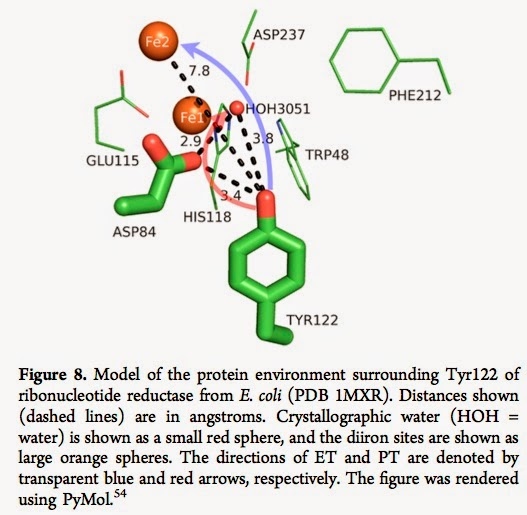
There are a diverse range of biomolecules where coupled electron-proton transfer plays a key role in their function. The electron transfer (ET) and proton transfer (PT) are usually spatially separated. [See blue and red arrows below].
There are fundamental questions about whether the transfer is concerted or sequential, adiabatic or non-adiabatic, and how important the protein environment (polar solvent) is.
Often short hydrogen bonds are involved and so the nuclear degrees of freedom need to be treated quantum mechanically, in order to take into account tunnelling and/or zero-point motion.
Diabatic states are key to understanding and theoretical model development.
Although there are some "schematic" theories, they involve some debatable approximations (e.g. Fermi's golden rule), and so there is much to be done, even at the level of minimal model Hamiltonians.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



No comments:
Post a Comment