Tautomerism in Porphycenes: Analysis of Rate-Affecting Factors
Piotr Ciąćka, Piotr Fita, Arkadiusz Listkowski, Michał Kijak, Santi Nonell, Daiki Kuzuhara, Hiroko Yamada, Czesław Radzewicz, and Jacek Waluk
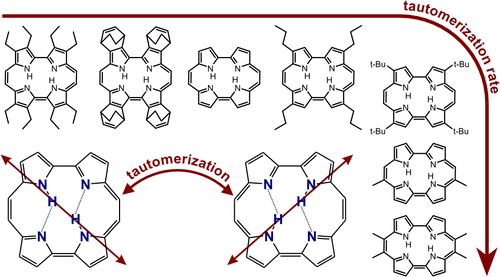
They look at nineteen different porphycenes, which means that R, the distance between the nitrogen atoms that donate and accept a proton varies.
[This is a testimony to the patience and skill of synthetic organic chemists to produce 19 different compounds.]
The rate of tautomerization [i.e. double proton transfer] can be measured my monitoring the time dependence of the fluorescence anisotropy because the transition dipole moment of the two tautomers is in different directions, as illustrated below, in the graphical abstract of the paper.
The key result is below: the rate of tautomerization [i.e. double proton transfer] versus R. Note the vertical scale varies by three orders of magnitude.
The natural explanation for this correlation is that as R decreases so does the energy barrier for double proton transfer. At least at the qualitative level this is captured by my simple diabatic state model for double proton transfer [which just appeared in J. Chem. Phys.]. However, my model only predicts a correlation is the ratio of the proton affinity of the donor with one and two protons on the donor does not change as one makes the chemical substitutions that change R.
(I think) all these experiments are done at room temperature in a solvent.
Two open questions concern whether the double proton transfer is sequential or concerted, and whether it is activated or involves tunnelling. At low temperatures in supercooled jets there is evidence of tunnel splitting and concerted transfer.





No comments:
Post a Comment