For my latest celebrity scientist speaking gig [at a small church youth group] my glamorous assistant [my wife] found a new demonstration to add to my repertoire, Elephants toothpaste. It is described in this Journal of Chemical Education paper.
Hydrogen peroxide is thermodynamically unstable. However, you can buy bottles of it and they will remain useful for months. It will slowly decompose into water and oxygen.
2 H
2O
2 → 2 H
2O + O
2
However, if you add some iron chloride it acts as a catalyst and increases the decomposition rate by a factor of a thousand. You will see some amount of "bubbling" due to the oxygen gas produced. If blood [which contains haemoglobin] is added the rate increases by a factor of a million. Even better, if you add the enzyme catalase, the rate increases by a factor of a billion. In the demonstration the catalase is present in the yeast that is added. Catalase is one of the fastest catalysts known. It performs an incredibly important biochemical function, that is essential to life existing. Hydrogen peroxide is a strong oxidant that could destroy many biomolecules. It is also an unwanted byproduct of many biochemical reactions. Biological systems use catalase to rapidly destroy the hydrogen peroxide before it can do harm.
The demonstration I did (and described in the JCE article) makes use of a dilute aqueous solution [a few per cent] of hydrogen peroxide. The spectacular video below makes use of a highly concentrated solution that is quite dangerous because it can cause chemical burns of the skin.
The above discussion follows the beautiful introduction to enzymes in chapter 11 of my favourite biochemistry text by Matthews, van Holde, Appling, and Anthony-Cahill
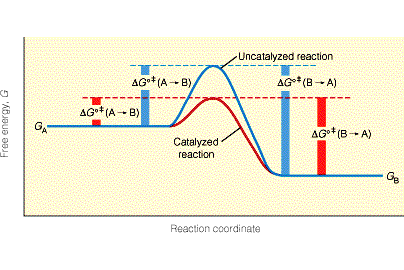
It contains the figure below, illustrating the key idea of how catalysts work: by lowering the energy barrier [the transition state] for a chemical reaction.
Subscribe to:
Post Comments (Atom)
A forgotten physicist: Amelia Frank (1906-1937)
In honour of International Women's Day, I bring to your attention a fascinating recent piece in The Conversation , Who was Amelia Frank?...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



Enzyme is a kind of catalytically active protein. Its catalytic efficiency is higher than inorganic catalysts. Except the general characteristics of the chemical catalyst, application of enzymes
ReplyDeleteThis is really a nice and informative, containing all information and also has a great impact on the new technology. Thanks for sharing it, Lasik Michigan
ReplyDelete