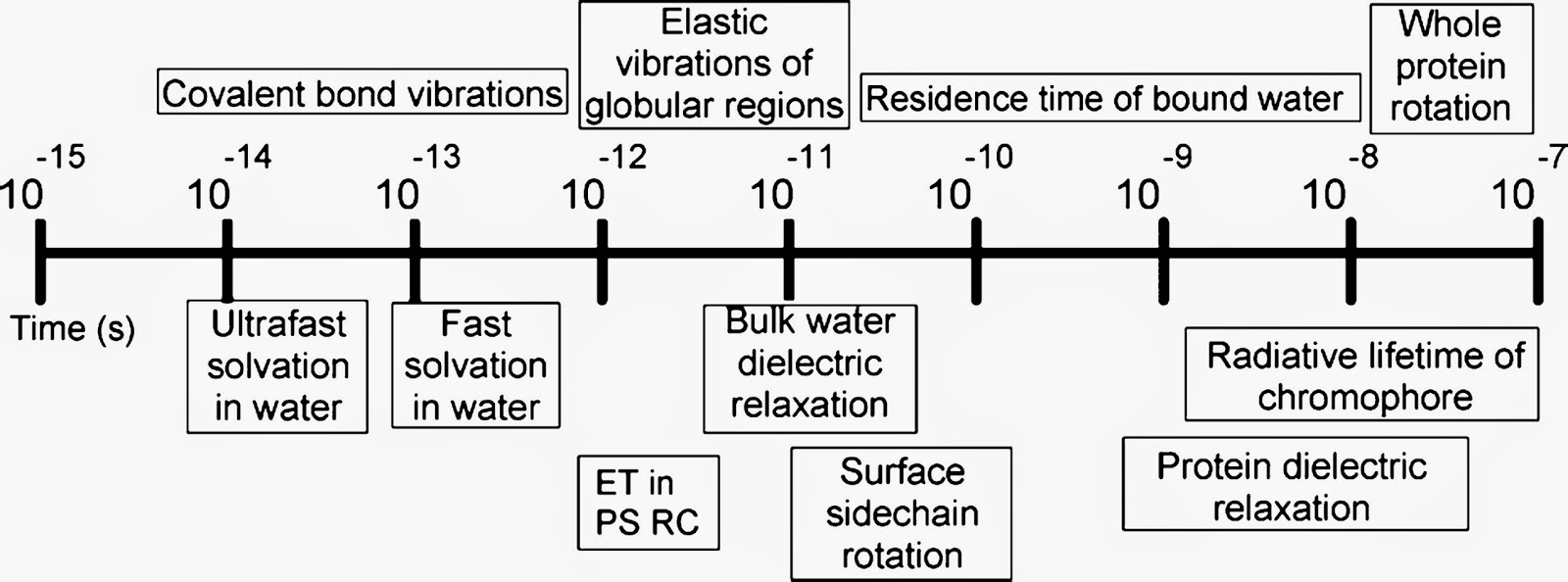
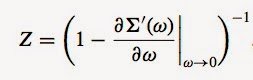I think we need more of these kind of frank discussions about scientific topics. I am slowly working through the answers. The most fascinating bit so far is Peter Wolynes inspiring response to Q9, including "I believe a young physicist who wants to work on any challenging problem in physics will eventually have to learn about glasses."
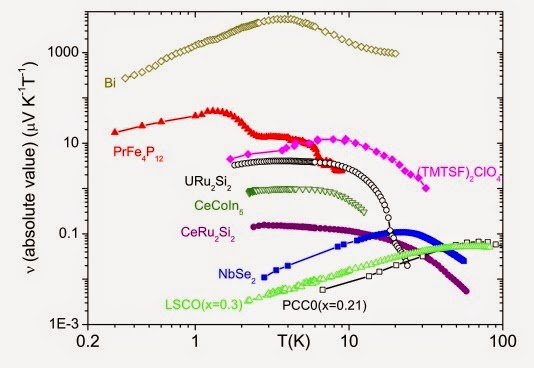
Q1) In your view, what are the most important aspects of the experimental data on the glass transition that any consistent theory explain? Is dynamical heterogeneity one of these core aspects?
Q2) Why should we expect anything universal in the behavior of glass-forming liquids? Is the glass-transition problem well defined?
Q3) In spin-glasses, the existence of a true spin-glass phase transition has been well established by simulations and experiments. Do you believe that a similar result will ever be demonstrated for molecular glasses?
Q4) Why are there so many different theories of glasses? What kind of decisive experiments do you suggest to perform to rule out at least some of them?
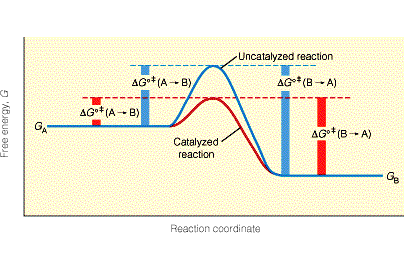
Q5) Can you briefly explain, and justify, why you believe your pet theory fares better than others? What, deep inside, are you worried about, that could jeopardize your theoretical construction?
Q6) In the hypothesis that Random First-Order Theory [RFOT] forms a correct skeleton of the theory of glasses, what is missing in the theoretical construction that would convince the community?
Q7) Exactly solvable mean-field glass models exhibit an extraordinary complexity requiring impressive mathematical tools to solve them.
Q8) In your view, do the recent ideas and experimental developments concerning jamming in granular media and colloids contribute to our understanding of molecular glasses, or are they essentially complementary?
Q9) If a young physicist asked you whether he or she should work on the glass problem in the next few years, would you encourage him or her and if so, which aspect of the glass problem would you recommend him or her to tackle?
Q10) In twenty years from now, what concepts, ideas or results obtained on the glass transition in the last twenty years will be remembered?
Q11) If you met an omniscient God and were allowed one single question on glasses, what would it be?I thank Peter Wolynes for bringing this to my attention.