I would say an ionic Hubbard model on the triangular lattice.
About a decade ago sodium cobaltate [NaxCoO2] was the "flavour of the month" when it came to strongly correlated electron materials. And then along came the iron pnictide superconductors....
The cobalt ions within a layer of the crystal structure form a triangular lattice and the sodium ions donate electrons to conducting layers. Hence, it is natural to consider a doped Hubbard model on a triangular lattice as the simplest possible effective Hamiltonian for these materials.
This led to numerous studies of this model. Today most studies of this model will also claim relevance to sodium cobaltates. I disagree.
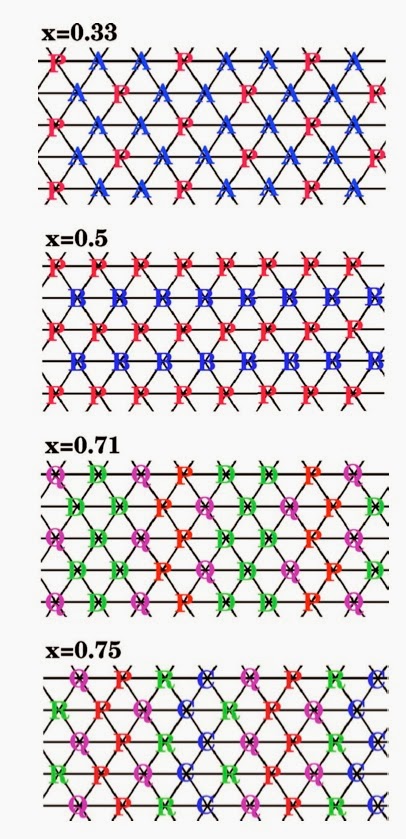
The sodium ions play a larger role that cannot be neglected. They actually modify the intra-layer electronic structure. Specifically, they spatially order in a manner dependent on the doping level.
This is unlike the case of the cuprates where the atoms between layers [and dopants] are merely spacers and do not change with doping.
Jaime Merino, Ben Powell, and I discussed this in a series of papers, discussed in an earlier post. In a 2006 PRB we considered the simple one band Hubbard model and pointed out that it could not describe all the cobaltates properties. The figure below shows how the spatial ordering of the sodium ions at different dopings produces different site energies on the triangular lattice.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...



No comments:
Post a Comment