I previously posted about how double proton transfer is a concrete example of a chemical reaction that can occur via either a concerted or sequential process?
Precisely defining this question and answering it is a subtle issue.
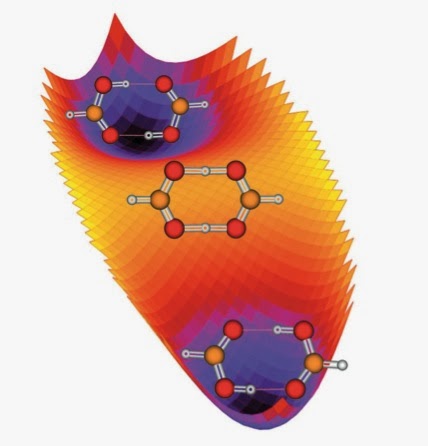
There is a nice classification of types of potential energy surfaces for double proton transfer, summarised on the website of Antonio Fernandez-Ramos. It is based on a very simple model potential energy surface described here and compared to surfaces from computational quantum chemistry [at the DFT level] here [source of the figures below].
There are three qualitatively different potential energy surfaces, depending on the strength of the coupling of the motion of the two protons.
(1) One transition state and two minima, as in the formic acid dimer;
(2) Two equivalent transition states, one maxima and two minima, as in the pyrazole dimer;
(3) Four transition states, one maxima and four minima, as in porphine.
I am pretty happy because I am developing a simple diabatic state model that captures all of the above cases.
Subscribe to:
Post Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...





No comments:
Post a Comment