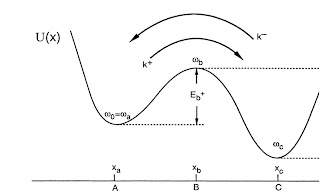
For "strong" friction the rate is controlled by "spatial diffusion" and given by
where gamma is the friction which can depend on frequency (i.e. be non-Markovian). This rate decreases monotonically with increasing friction.For "weak" friction the rate is controlled by "energy diffusion" and increases monotonically with increasing friction.
Interpolating between the two regimes is difficult. But, the important point is there should be a Kramers turnover in the prefactor. The dependence on friction is shown below
Observing this Kramers' turnover has been a bit of a "holy grail". One way one can tune the friction is by varying the viscosity (or diffusion constant) of the fluid environment by varying its density via pressure. The data below shows the rate of photoisomerisation of trans-stillbene as a function of the diffusion constant.
It appears to show the turnover but there are quite a few questions about the details.... It seems the activation energy varies with pressure and the barrier frequency (omega_b) varies with temperature (see this 1995 paper).
A somewhat accessible discussion of all of this in a 1990 Physics Today article, Chemical Dynamics in Solution by Fleming and Wolynes.







No comments:
Post a Comment