In the Intermediate Biophysics class meeting today we agreed to start reading through Philip Nelson's excellent book, Biological Physics: Energy, Information, and Life.
Each chapter begins with a biological question, and a terse slogan encapsulating a physical idea relevant to the question. Chapter 1 begins with:
Biological question: How can living organisms be so highly ordered?
Physical idea: The flow of energy can leave behind increased order.
He introduces the idea of free energy as the useful energy or quality of energy. The distinction between high and low quality energy is a matter of order or organisation.
Living beings consume order not energy.
Free energy transduction is what the biosphere does to create order.
The figure below is a nice way to illustrate transduction of free energy.
A machine uses osmotic flow to convert disorder (random molecular motion) into work in the upper part of the figure.
In the lower part of the figure doing work pulling on the weight increases the order in the system, i.e., the sugar solution becomes more concentrated (reverse osmosis).
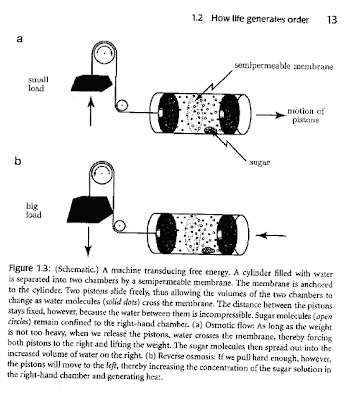




That's a good figure Ross, it provides a good conceptual illustration of getting work, or perhaps even order, from the increasing disorder in one part of the system. Another illustration I personally like is the lipid bi-layer, which is apparently highly ordered and obviously very abundant in any organism. However if we consider a single lipid in solution, we can see that the resulting hydration shell is actually more ordered than the solution around a lipid bi-layer. Therefore, the formation of ordered lipid bi-layers increases the disorder of the solution, resulting in a net increase in entropy and holding true to the second law of thermodynamics.
ReplyDeleteTalk to you soon, Alexander.