
Thinking in the alternative resonating valence bond picture there will be three alternative Lewis structures
O
||
C -R
|
L
O-
|
C -R
|
L+
O-
|
C -R+
|
L
The extent to which the lower two structures contribute will increase the C-O bond length and reduce the C-O stretch frequency.
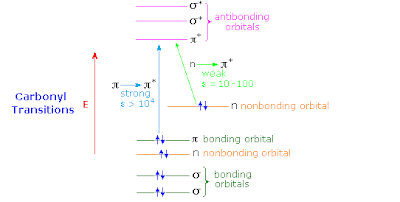
It should be possible to describe the low lying excited states in terms of a complete active space with 4 electrons in 3 orbitals (a pi* orbital on the C=O bridge, and a pi orbital on the left and right fragments).




No comments:
Post a Comment