Emergence is one of the most important concepts in the sciences: from physics to biology to sociology. Most of the big questions in science involve emergence. Yet there is no consensus about what emergence is, how to define it, or why it matters. This is my attempt to clarify some of the important issues and questions. For reasons of brevity, I give no references and only a few examples. They can come later. Here I am trying to take a path that is intermediate between the precision of philosophers and the looseness of condensed matter physicists' discussion of emergence. My goals are clarity and brevity.
Characteristics of emergent phenomena
Consider a system that is composed of many interacting parts. If the properties of the system are compared with the properties of the individual parts, a property of the whole system is an emergent property if it has the following characteristics.
1. Novelty
An emergent property of the system is a property that is not present in the individual parts of the system.
2. Modification of parts
An emergent property of the system is associated with a modification of the properties of and the relationships between the parts of the system.
3. Universality
An emergent property is universal in the sense that it is independent of many of the details of the parts. As a consequence, there are many systems that can have the emergent property.
4. Irreducibility
An emergent property cannot be reduced to properties of the parts.
5. Limited predictability
An emergent property is difficult to predict solely from knowledge of the properties of the parts and how they interact with one another.
Here are a few issues to consider about the five characteristics above.
First, “emergent property” could possibly be replaced with emergent phenomenon, object, or state.
Second, for each of the five characteristics is it necessary and/or sufficient for the system property to be emergent?
Third, one of the most contested characteristics concerns predictability. “Difficult to predict” is sometimes replaced with “impossible”, “almost impossible”, “extremely difficult”, or “possible in principle, but impossible in practice.” After an emergent property has been observed sometimes it can be understood in terms of the properties of the parts. An example is the BCS theory of superconductivity, which provided a posteriori, rather than a priori, understanding. A keyword in the statement above is “solely”.
Examples of properties of a system that are not emergent are volume, mass, charge, and number of atoms. These are additive properties. The property of the system is simply the sum of the properties of the parts.
Scales and hierarchies
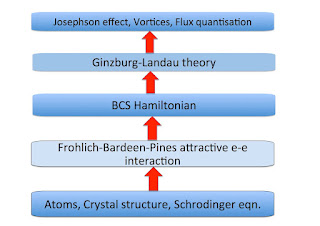
Central to emergence is the idea of different scales. Emergent properties only occur when scales become larger. Scales that are simply defined, and might be called extrinsic, are the number of parts, length scale, and time scale. A more subtle scale, which might be called intrinsic, is a scale associated with the emergent property. This emergent scale is intermediate between that of the parts and that of the whole system.
Emergent scales lead naturally to hierarchies, such as those associated with different scientific disciplines, as shown below. Hierarchies also occur within individual disciplines.
At each level there are distinct phenomena, concepts, theories, and scientific methods.
Another important scale is that of complexity. Generally, as one goes up the hierarchy one says that the level of complexity increases. Giving a precise version of such statements is not simple.
Complexity
Simple rules can lead to complex behaviour. This is nicely illustrated by cellular automata. It is also seen in other systems with emergent properties. For example, the laws describing the properties of electrons and ions in a crystal or a large molecule are quite simple (Schrodinger’s equation plus Coulomb’s law). Yet from these simple rules, complex phenomena emerge: all of chemistry and condensed matter physics!
There is no agreed universal measure for the complexity of a system or with many components. One possibility is the Kolmogorov measure. Using such measures to elucidate emergence, such as how complexity changes with other scales, is an important challenge.
Other issues
There are a host of other issues and topics that enter discussions about emergence. Some of these are of a more philosophical nature. Here I just list them: robustness, quality vs. quantity, objective vs. subjective, universality vs. particularity, ontology vs. epistemology, discontinuities, incommensurability, theory reduction, asymptotic singularities, top-down causation, supervenience, differentiation and integration (not calculus) of system parts, reductionism, foundationalism, fundamentalism, strong versus weak emergence, and criteria for theory acceptance.
Discussion of some of these issues can be quite abstract but to make the discussion above more precise they may need to be considered.
Emergence is relevant to practical matters such as scientific strategy, priorities, allocation of resources, and our dispositions as scientists. Too often views on these issues are implicit and not reflected upon.
The practical matter of scientific strategy
When studying a system, the first choice that must be made is what scale or scales to focus on. For example, in materials science, the options range from the atomic scale to the macroscopic. This choice determines the tools and methods, both experimental and theoretical, that can be used to study the system. In different words, the scientist is making a choice of ontology: the object they choose to study. This then determines epistemology: the concepts, theories, and organising principles a scientist may use or hope to discover. Effective theories and toy models enter here.
When systems have been studied by a range of methods and at a range of scales, a challenge is the synthesis of the results of these studies. Value-laden judgements are made about the priority, importance, and validity of such attempts at synthesis. Often synthesis is relegated to a few sentences in the introductions and conclusions of papers.
For known systems and emergent properties, there is the possibility of creating new methods and probes to investigate them at appropriate scales.
New systems can be created and investigated in the hope of discovering new emergent properties (e.g., new states of matter) or more modestly, that manifest an emergent property that is more amenable to scientific study or technological application.
As emergent properties involve multiple scales they are often of interest to and amenable to study by more than one scientific discipline. This creates opportunities and challenges for interdisciplinary collaboration.
Individual scientists must and do make decisions about the relative priority of the different strategies outlined above. Research groups, departments, institutions, professional societies, and funding agencies must and do also make decisions about such priorities. The decision outcomes are also emergent properties of a system with multiple scales from that of the individual scientist to global politics. I claim that too often these weighty decisions are made implicitly, rather than explicitly following debate and deliberation.
The disposition of the scientist
All scientists are human. In our professional life, we have hopes, aspirations, values, fears, attitudes, expectations, and prejudices. These are shaped by multiple influences from the personal to the cultural to the institutional. We should reflect on the past century of our study of emergent systems from physics to biology to sociology. If we honestly evaluate our successes and failures I think this may lead us to have certain dispositions that are interrelated.
Humility. There is so much we do not understand. Furthermore, we fail abjectly at predicting emergent properties. This is not surprising. Unpredictability is one of the characteristics of emergent properties. There is a hubris associated with grand initiatives such as “the theory of everything”, the Human Genome Project, “materials by design”, and macroeconomic modelling.
Expect surprises. There are many exciting discoveries waiting. They will be found by curiosity and serendipity.
Wonder. Emergent phenomena are incredibly rich and beautiful to behold, from physics to biology to sociology. Furthermore, the past century has seen amazing levels of understanding. But this is a “big picture” and “coarse-grained” understanding, not the description that the reductionists lust for and claim possible.
Realistic expectations. Given the considerations above I think we should have modest expectations of the levels of understanding possible, and what research programs, from that of individual scientists to billion-dollar initiatives, can achieve. We need to stop the hype. Modest expectations are particularly appropriate with respect to our ability to control emergent properties.
The holy grail
“The philosophers have only interpreted the world, in various ways. The point, however, is to change it.”
Karl Marx
Understanding complex systems with emergent properties is an ambitious scientific challenge. This enterprise has intrinsic intellectual merits. But a whole other dimension and challenge is to use this understanding to modify, manipulate, and control the properties of systems with emergent properties. This enticing prospect appeals to technologists, activists, and governments. Such promises feature prominently in grant applications, press releases, and reports from funding agencies. Diverse examples of this control goal include chemical modification of known superconductors to produce room-temperature superconductivity, drug design, social activism, the leadership of business corporations, and governments attempting to manage the economy.
However, we should honestly reflect on decades of “scientifically informed” and “evidence-based” initiatives in materials science, medicine, poverty alleviation, government economic policy, business management, and political activism. Unfortunately, the fruit from these initiatives is disappointing, particularly compared to what has often been promised.
My goal is not to promote despair but rather to prevent it. With more realistic expectations, based on reality rather than fantasy, we are more likely to make significant progress in finding ways to make some progress (albeit modest but worthwhile) in learning how to manipulate these complex systems.
This post contains many claims that require discussion, refinement or abandonment. I welcome suggestions on how to improve these ideas.