Sometimes I bemoan the decline of scholarship in science, and in academia more broadly. About six years ago I posted about Ph.D's without scholarship, which generated a lot of comments.
This decline is reflected in a range of phenomena: hype, making hiring and promotion decisions based on metrics rather than actual scientific achievements, people writing more papers than they read, "review" articles merely listing references rather than providing critical analysis,...
But, this is all negative, it is what scholarship is not, ... what does real scholarship look like?
I think classic books give a feel for what scholarship is all about. For example, Eisenberg and Kauzmann on Water, Ashcroft and Mermin, Hewson's Kondo Problem, Coulson's Valence, and Mott's monographs. Consider the Oxford Classic Texts in the Physical Sciences.
Similarly, I am challenged by some of the monographs that some humanities colleagues produce. (For example, Stephen Gaukroger's three volumes on science and the shaping of modernity.)
But, today I just don't see people in physics and chemistry producing books like the above.
Am I missing something?
There is certainly a subjective element. Here are a few possible ingredients to real scholarship.
1. Acknowledge the past.
Every problem, achievement, and discipline actually normally has a long history.
Even Newton said he was standing on the shoulders of giants.
2. Acknowledge and engage with the work of others and different points of view.
3. Acknowledge ambiguity, complexity, and controversy.
4. Comprehensive.
A wide range of topics are considered. The focus is not just narrow.
5. Synthesis and coherence.
A wide range of ideas, topics, and techniques are brought together.
6. Lucidity.
Do you think scholarship is declining?
What do you think are the key ingredients?
Thursday, April 28, 2016
Tuesday, April 26, 2016
Low temperature physics without nuclear weapons
Liquid 3He is amazing stuff. Below temperatures of a few hundred milliKelvin it forms a model (and the original inspiration for) Landau Fermi liquid. Furthermore, below about 1 mK it forms two different superfluid states, involving Cooper pairs in a spin triplet state. This is the model case for unconventional superconductivity.
Liquid 3He is actually of great practical use since it the crucial ingredient of dilution refrigerations that allow cooling from a few Kelvin to temperatures as low milliKelvin.
But where do labs get 3He from?
Well, it is a very useful by-product of nuclear weapons production.
Currently, the scientific community (which consumes only about 1% of the supply) is experience supply problems and dramatic price increases (a 15-fold increase between 2004 and 2010).
Why is this happening?
Thankfully, we are cutting back on nuclear weapons production!
One practical way to solve this problem is to develop alternative materials for ultra-low temperature refrigeration; one possibility is by adiabatic demagnetisation. Indeed, this is the method that was first developed in the 1930s using paramagnetic salts to achieve temperatures below about 0.3 K (and was the basis of the 1949 Nobel Prize in Chemistry) and is the basis for nice undergraduate problems in thermodynamics and statistical mechanics. Simply the entropy is a function of B/T (where B is the magnetic field and T the temperature). One cools the system down in a fixed magnetic field, then adiabatic isolates it and reduces the magnetic field slowly. In the last step the entropy must not change and so the temperature must decrease. (This is shown as the red horizontal arrow in the figure below). This is also known as the magnetocaloric effect. The problem is that most paramagnetic materials are insulators and one would prefer to have a metallic material that is a good thermal conductor and can be "machined".
I learnt some of this from an interesting paper (that I actually looked at in preparing an undergraduate thermodynamics lecture about Maxwell relations).
Large magnetocaloric effect and adiabatic demagnetization refrigeration with YbPt2Sn
Dongjin Jang, Thomas Gruner, Alexander Steppke, Keisuke Mitsumoto, Christoph Geibel and Manuel Brando
The authors mention some basic unanswered science questions about why this material is a good candidate. Specifically, why is the Kondo temperature (associated with interaction of the magnetic moments of the Yb3+ ions with the conduction electrons) and the inter-ion magnetic interactions so low? This ensures that the spins act essentially like non-interacting spins (with a large entropy) down to less than 1 K.
A key figure is below, showing the entropy versus temperature at several different magnetic fields.
Liquid 3He is actually of great practical use since it the crucial ingredient of dilution refrigerations that allow cooling from a few Kelvin to temperatures as low milliKelvin.
But where do labs get 3He from?
Well, it is a very useful by-product of nuclear weapons production.
Currently, the scientific community (which consumes only about 1% of the supply) is experience supply problems and dramatic price increases (a 15-fold increase between 2004 and 2010).
Why is this happening?
Thankfully, we are cutting back on nuclear weapons production!
One practical way to solve this problem is to develop alternative materials for ultra-low temperature refrigeration; one possibility is by adiabatic demagnetisation. Indeed, this is the method that was first developed in the 1930s using paramagnetic salts to achieve temperatures below about 0.3 K (and was the basis of the 1949 Nobel Prize in Chemistry) and is the basis for nice undergraduate problems in thermodynamics and statistical mechanics. Simply the entropy is a function of B/T (where B is the magnetic field and T the temperature). One cools the system down in a fixed magnetic field, then adiabatic isolates it and reduces the magnetic field slowly. In the last step the entropy must not change and so the temperature must decrease. (This is shown as the red horizontal arrow in the figure below). This is also known as the magnetocaloric effect. The problem is that most paramagnetic materials are insulators and one would prefer to have a metallic material that is a good thermal conductor and can be "machined".
I learnt some of this from an interesting paper (that I actually looked at in preparing an undergraduate thermodynamics lecture about Maxwell relations).
Large magnetocaloric effect and adiabatic demagnetization refrigeration with YbPt2Sn
Dongjin Jang, Thomas Gruner, Alexander Steppke, Keisuke Mitsumoto, Christoph Geibel and Manuel Brando
The authors mention some basic unanswered science questions about why this material is a good candidate. Specifically, why is the Kondo temperature (associated with interaction of the magnetic moments of the Yb3+ ions with the conduction electrons) and the inter-ion magnetic interactions so low? This ensures that the spins act essentially like non-interacting spins (with a large entropy) down to less than 1 K.
A key figure is below, showing the entropy versus temperature at several different magnetic fields.
Friday, April 22, 2016
KITP seminars online
A wonderful thing about the web is that now there is so much material online. A pioneer in putting all their seminars and colloquia online is the KITP at Santa Barbara. I know some people who regularly watch seminars (both old and recent). Others do not know it exist. This is a particularly valuable resource for students and those of us in distant countries.
I have to confess that until yesterday I have never actually watched a talk; just occasionally skimmed some slides. Generally I find I don't have the patience to watch talks online. I just seem to prefer to look at papers. However, yesterday I was forced to do this because at the weekly UQ condensed matter theory group meeting we watched a nice talk by Antoine Georges on Hund's metals. Although, I have read and blogged about some of the relevant papers, I really found it helpful seeing what was highlighted and going through the material at a "slow pace". Hopefully, I will do this more often.
What do you think about online talks or lectures? How often do you watch them? Are there any that you would particularly recommend?
I have to confess that until yesterday I have never actually watched a talk; just occasionally skimmed some slides. Generally I find I don't have the patience to watch talks online. I just seem to prefer to look at papers. However, yesterday I was forced to do this because at the weekly UQ condensed matter theory group meeting we watched a nice talk by Antoine Georges on Hund's metals. Although, I have read and blogged about some of the relevant papers, I really found it helpful seeing what was highlighted and going through the material at a "slow pace". Hopefully, I will do this more often.
What do you think about online talks or lectures? How often do you watch them? Are there any that you would particularly recommend?
Thursday, April 21, 2016
Cost benefit analysis of administrative policies
Administrators and senior management seem to love coming up with new policies and procedures for everything.
These are designed to make things "better".
However, a colleague recently emphasised to me that each one of these initiatives should be subject to a cost-benefit analysis. This is a point I have also heard made by my UQ law colleague, James Allen, author of a provocative essay about Australian universities.
Consider the follow examples:
* requiring grant applications to provide more information (whether reports on previous grants, details about university policies, longer project descriptions, relevance to society, ....)
* more details in course profiles
* larger committees to ensure more input, consultation, representation of diversity, accountability, and expertise
* procedures and policies to increase transparency and accountability
* broadening eligibility criteria so more people can apply for a particular grant or fellowship program.
Every one of these initiatives has benefits.
So why might they be a bad idea?
One needs to consider the cost, particularly the opportunity cost.
Specifically, if instead of faculty spending time on these tasks what might they spend time on instead?
Mentoring graduate students and postdocs, research, preparing higher quality lectures, ....
Sometimes senior faculty simply move these tasks to junior faculty, graduate students, or junior administrative staff. However, that has a cost too. Implementing all these initiatives requires more admin staff; money that could be spent instead on hiring more faculty...
It is not just time and money. All this admin. takes mental space and sometimes reduces morale, which in the end leads to reduced productivity.
These are designed to make things "better".
However, a colleague recently emphasised to me that each one of these initiatives should be subject to a cost-benefit analysis. This is a point I have also heard made by my UQ law colleague, James Allen, author of a provocative essay about Australian universities.
Consider the follow examples:
* requiring grant applications to provide more information (whether reports on previous grants, details about university policies, longer project descriptions, relevance to society, ....)
* more details in course profiles
* larger committees to ensure more input, consultation, representation of diversity, accountability, and expertise
* procedures and policies to increase transparency and accountability
* broadening eligibility criteria so more people can apply for a particular grant or fellowship program.
Every one of these initiatives has benefits.
So why might they be a bad idea?
One needs to consider the cost, particularly the opportunity cost.
Specifically, if instead of faculty spending time on these tasks what might they spend time on instead?
Mentoring graduate students and postdocs, research, preparing higher quality lectures, ....
Sometimes senior faculty simply move these tasks to junior faculty, graduate students, or junior administrative staff. However, that has a cost too. Implementing all these initiatives requires more admin staff; money that could be spent instead on hiring more faculty...
It is not just time and money. All this admin. takes mental space and sometimes reduces morale, which in the end leads to reduced productivity.
Monday, April 18, 2016
Incorporating scientist biographies into lectures
A few years ago I decided I wanted to include brief biographies of relevant great scientists in my undergraduate lectures. I posted (5 years ago!) about how I started with Landau but I lost momentum. This year I have put more effort into it. I just taught my second year undergraduate thermo class about Gibbs free energy and so I profiled Gibbs.
In solid state physics I have profiled Drude, Sommerfeld, von Laue, and Bloch.
I have found this quite enjoyable for myself and hopefully for the students. I have learnt quite a bit, just by reading the relevant Wikipedia pages. It also introduces students to the human dimension of science. For example, Drude died by suicide and so it is a good opportunity to flag mental health issues. Sommerfeld was a mentor of many great scientists. von Laue actively opposed the Deutsche Physik of the Nazis. Bloch was the first Director General of CERN.
Has anyone else experience at doing similar things? Any suggestions?
In solid state physics I have profiled Drude, Sommerfeld, von Laue, and Bloch.
I have found this quite enjoyable for myself and hopefully for the students. I have learnt quite a bit, just by reading the relevant Wikipedia pages. It also introduces students to the human dimension of science. For example, Drude died by suicide and so it is a good opportunity to flag mental health issues. Sommerfeld was a mentor of many great scientists. von Laue actively opposed the Deutsche Physik of the Nazis. Bloch was the first Director General of CERN.
Has anyone else experience at doing similar things? Any suggestions?
Friday, April 15, 2016
Start the mechanics of producing your thesis now (not in the frantic last days!)
Producing a thesis (Ph.D, Masters, or undergraduate honours) is a monumental task that can create significant stress. Here I am just going to focus on the mechanics of producing the final document, not the greater challenge of producing the intellectual content.
In most cases there is a deadline, whether imposed by the program, funding running out, or (hopefully) a fixed date to start a job. For many students there is a big rush at the end featuring very long hours, missing "life", neglecting family and sometimes health, exhaustion, anxiety, ...
These problems are compounded if one starts "writing" and producing the thesis document at the very end, with the final deadline looming. Furthermore, this can be much slower and more frustrating if you have to do some of the mechanics (e.g. ordering and numbering references) by hand or at least learn to use software to do it automatically. Compared to 30 years ago, the mechanics is now so much easier because of software that automates many of the tedious tasks such as ordering and numbering.
Let me encourage you to start now on the mechanics.
First, you need to decide on and learn relevant software and start using it every step of the way. Talk to other students (just finished or finishing) and postdocs to find out what they used, and the relative merits.
Here are some concrete issues to consider.
Keeping track of references.
From day one you will start downloading (and hopefully reading) papers. By the end you will (hopefully) have hundreds of references. How are you going to sort them and organise them? If when you start writing you just have hundreds of PDFs in a folder on your computer, life is going to be difficult if you have to start looking at them one by one to find some particular reference you need.
I use the wonderful program Papers. Others like Endnote. The main thing is to find something that works for you, including interfacing smoothly with your word processor program.
Ordering and formatting of references.
For LaTeX, BibTeX does this nicely, provided you learn how to get it to produce the desired format.
Generating a bibliography.
Papers can easily produce a .bib file for BibTex.
Producing graphs and schematics and incorporating figures in the document.
On a previous post, commenters on a post discussed useful freeware for plotting data.
Spell and grammar check.
This is particularly important for students whose English is weak or are dyslexic. Advisors and examiners really get irritated by too many typos. I have never found a decent spell checker for LaTex. Any recommendations?
Thesis template.
This should include all the relevant sections from cover page to lists of figures to acknowledgements. It should automatically order and number everything: pages, chapters, figures, tables, equations, references, ....
Keep it simple.
Avoid personalised versions of templates and software (e.g. LaTeX macros), either from you or someone else. My limited experience is that these are often not as portable as hoped/claimed and lead to small bugs that can waste precious time trying to fix.
Backup everything regularly.
This is so easy but it is amazing and disappointing how I still hear of students losing work. Dropbox, cheap portable Terabyte drives, and Time Machine on Mac's make this inexcusable.
Don't just backup the thesis document, but all your data, codes, references, ....
Hard drives do crash and laptops do get lost or stolen...
Practise writing now.
Writing is hard work. Don't wait until the final stages of the thesis to start to learn how to write. It is too late.
Getting feedback from your advisor now.
Again during the final stages is not to learn how she likes things formatted or how to write figure captions.
What software would you recommend or avoid?
In most cases there is a deadline, whether imposed by the program, funding running out, or (hopefully) a fixed date to start a job. For many students there is a big rush at the end featuring very long hours, missing "life", neglecting family and sometimes health, exhaustion, anxiety, ...
These problems are compounded if one starts "writing" and producing the thesis document at the very end, with the final deadline looming. Furthermore, this can be much slower and more frustrating if you have to do some of the mechanics (e.g. ordering and numbering references) by hand or at least learn to use software to do it automatically. Compared to 30 years ago, the mechanics is now so much easier because of software that automates many of the tedious tasks such as ordering and numbering.
Let me encourage you to start now on the mechanics.
First, you need to decide on and learn relevant software and start using it every step of the way. Talk to other students (just finished or finishing) and postdocs to find out what they used, and the relative merits.
Here are some concrete issues to consider.
Keeping track of references.
From day one you will start downloading (and hopefully reading) papers. By the end you will (hopefully) have hundreds of references. How are you going to sort them and organise them? If when you start writing you just have hundreds of PDFs in a folder on your computer, life is going to be difficult if you have to start looking at them one by one to find some particular reference you need.
I use the wonderful program Papers. Others like Endnote. The main thing is to find something that works for you, including interfacing smoothly with your word processor program.
Ordering and formatting of references.
For LaTeX, BibTeX does this nicely, provided you learn how to get it to produce the desired format.
Generating a bibliography.
Papers can easily produce a .bib file for BibTex.
Producing graphs and schematics and incorporating figures in the document.
On a previous post, commenters on a post discussed useful freeware for plotting data.
Spell and grammar check.
This is particularly important for students whose English is weak or are dyslexic. Advisors and examiners really get irritated by too many typos. I have never found a decent spell checker for LaTex. Any recommendations?
Thesis template.
This should include all the relevant sections from cover page to lists of figures to acknowledgements. It should automatically order and number everything: pages, chapters, figures, tables, equations, references, ....
Keep it simple.
Avoid personalised versions of templates and software (e.g. LaTeX macros), either from you or someone else. My limited experience is that these are often not as portable as hoped/claimed and lead to small bugs that can waste precious time trying to fix.
Backup everything regularly.
This is so easy but it is amazing and disappointing how I still hear of students losing work. Dropbox, cheap portable Terabyte drives, and Time Machine on Mac's make this inexcusable.
Don't just backup the thesis document, but all your data, codes, references, ....
Hard drives do crash and laptops do get lost or stolen...
Practise writing now.
Writing is hard work. Don't wait until the final stages of the thesis to start to learn how to write. It is too late.
Getting feedback from your advisor now.
Again during the final stages is not to learn how she likes things formatted or how to write figure captions.
What software would you recommend or avoid?
Wednesday, April 13, 2016
How Ashcroft and Mermin quickly became irrelevant and then relevant
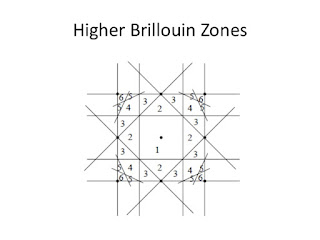
This week in my Solid State Physics class I taught covered weak periodic potentials (including higher Brilloiun zones and Fermi surface reconstruction) and the tight binding model. I closely follow chapters 9 and 10 in Aschroft and Mermin, which was published in 1975.
This topic is somewhat iconic in that it features on the front and back cover of the book.
I think for the first time I understood the higher Brilloiun zones (rather than being overwhelmed by the geometrical complexity) and how this leads to the complex hole Fermi surfaces for metals of valence 2, 3, and 4. The key to visualising this better is just to do the problem in two dimensions first.
This got me wondering: why do we teach this stuff to students?
First, there is the intellectual beauty of the subject: how simple analytical and geometrical models can capture the complex band structures and Fermi surfaces of elemental metals.
However, today almost no one cares about elemental metals, or at least does research on them.
Note that most physics undergraduates and graduates don't ever take a course on nuclear physics. My department does not even teach one! Yet, the subject is a beautiful one and of great historical importance (both intellectually and politically!). The reason for this is that there is now very little research in basic nuclear physics. (I think this is a bad thing, but that is another story..)
But, metal physics is different.
A compelling reason I teach it in detail is that it provides a foundation to understand so much condensed matter research today: particularly how strongly correlated electron materials do (and do not) deviate from the Fermi liquid paradigm. Otherwise, I think Ashcroft and Mermin type courses would have been eventually sent off to the electrical engineering and materials engineering departments.
One can argue that almost happened. For the decade (1975-1985) following publication of the book, the content (not just on metals but also superconductivity) must have been largely of historical interest or considered only of interest to those working in "applied physics". But, the discovery of superconducting cuprates, heavy fermions, organic charge transfer salts, and iron pnictides, changed all that....
This topic is somewhat iconic in that it features on the front and back cover of the book.
I think for the first time I understood the higher Brilloiun zones (rather than being overwhelmed by the geometrical complexity) and how this leads to the complex hole Fermi surfaces for metals of valence 2, 3, and 4. The key to visualising this better is just to do the problem in two dimensions first.
This got me wondering: why do we teach this stuff to students?
First, there is the intellectual beauty of the subject: how simple analytical and geometrical models can capture the complex band structures and Fermi surfaces of elemental metals.
However, today almost no one cares about elemental metals, or at least does research on them.
Note that most physics undergraduates and graduates don't ever take a course on nuclear physics. My department does not even teach one! Yet, the subject is a beautiful one and of great historical importance (both intellectually and politically!). The reason for this is that there is now very little research in basic nuclear physics. (I think this is a bad thing, but that is another story..)
But, metal physics is different.
A compelling reason I teach it in detail is that it provides a foundation to understand so much condensed matter research today: particularly how strongly correlated electron materials do (and do not) deviate from the Fermi liquid paradigm. Otherwise, I think Ashcroft and Mermin type courses would have been eventually sent off to the electrical engineering and materials engineering departments.
One can argue that almost happened. For the decade (1975-1985) following publication of the book, the content (not just on metals but also superconductivity) must have been largely of historical interest or considered only of interest to those working in "applied physics". But, the discovery of superconducting cuprates, heavy fermions, organic charge transfer salts, and iron pnictides, changed all that....
Tuesday, April 12, 2016
The problem of self citation
I recently read a couple of review articles about topics I am trying to learn about. What was really striking was how much the authors cited their own work. Indeed, in one review the majority of references were those of the author!
Sometimes it is very appropriate that authors cite their own work. This previous work is relevant and the current work builds on the foundations of earlier work by the author. Sometimes it gives necessary background and more detail for understanding the current work.
However, there are bad reasons for authors to cite themselves.
1. It is a cynical exercise in boosting their own citation metrics.
2. They actually don't care what others are doing.
3. They don't want to acknowledge other work which contradicts or criticises their own, or at least presents an alternative picture of the problem.
Most people are concerned about 1, and this is certainly a legitimate concern, particularly as metric madness increases.
My focus is more on 3.
When I see this predominance of self-citation in an area I know little about my questions are:
- Does anyone else care about this topic and/or the authors approach?
- Is the author hiding something?
Sometimes it is very appropriate that authors cite their own work. This previous work is relevant and the current work builds on the foundations of earlier work by the author. Sometimes it gives necessary background and more detail for understanding the current work.
However, there are bad reasons for authors to cite themselves.
1. It is a cynical exercise in boosting their own citation metrics.
2. They actually don't care what others are doing.
3. They don't want to acknowledge other work which contradicts or criticises their own, or at least presents an alternative picture of the problem.
Most people are concerned about 1, and this is certainly a legitimate concern, particularly as metric madness increases.
My focus is more on 3.
When I see this predominance of self-citation in an area I know little about my questions are:
- Does anyone else care about this topic and/or the authors approach?
- Is the author hiding something?
Saturday, April 9, 2016
A helpful question to include in any student homework assignment
It is good to encourage students to be reflective in a concrete way about how they are going in a course. It is even better if the teacher knows what they are thinking.
I recently stumbled across the following idea. Include the following questions on a homework problem set.
(b) What is the concept that you understand the least?
(c) What specific action are you going to take to address (b)?
(d) List some of the skills required in this course. Which one do you think that you most need to improve? How might you do that?
I recently did this for my undergraduate solid state physics course.
I found the answers to (a) interesting. Sometimes the things that we think are interesting or boring are not necessarily the same as students. Many of my students said they really liked learning about crystal structures.
(c) is good because it makes the student actually reflect on what they might do and putting it down in writing hopefully creates some pressure and accountability for them to do it.
I think (d) is particularly important for my course. One reason why students find it so difficult is that it not only draws together many areas of physics (quantum, stat. mech., electromagnetism), but also uses a range of mathematics skills (calculus, sketching graphs, series expansions,...), and general scientific skills (model building, comparing experiment and theory, approximations, critical thinking, estimating orders of magnitude,...., keeping track of physical units, ....
Do you have any experience with similar exercises to encourage students to be self-reflective and take responsibility for addressing weaknesses?
Thursday, April 7, 2016
Review of nuclear quantum effects in water
Chemical Reviews just published an article
Nuclear Quantum Effects in Water and Aqueous Systems: Experiment, Theory, and Current Challenges
Michele Ceriotti, Wei Fang, Peter G. Kusalik, Ross H. McKenzie, Angelos Michaelides, Miguel A. Morales, and Thomas E. Markland
(Trivia: 4 out of 7 authors have a surname beginning with M!)
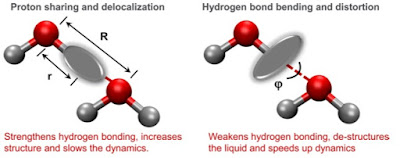
One of the unifying themes in the review is that of competing quantum effects, illustrated above.
This article is a direct outcome of the NORDITA program, "Water - the most anomalous liquid" that I attended about 18 months ago.
Other reviews from the program will appear together in a special issue of the journal.
I must confess I was skeptical that we were going to be able to pull off these reviews, written by large teams of busy and opinionated individuals.
For ours, we are greatly in debt to Tom Markland for his perseverance and leadership.
We welcome any comments about the contents of the review.
Nuclear Quantum Effects in Water and Aqueous Systems: Experiment, Theory, and Current Challenges
Michele Ceriotti, Wei Fang, Peter G. Kusalik, Ross H. McKenzie, Angelos Michaelides, Miguel A. Morales, and Thomas E. Markland
(Trivia: 4 out of 7 authors have a surname beginning with M!)
One of the unifying themes in the review is that of competing quantum effects, illustrated above.
This article is a direct outcome of the NORDITA program, "Water - the most anomalous liquid" that I attended about 18 months ago.
Other reviews from the program will appear together in a special issue of the journal.
I must confess I was skeptical that we were going to be able to pull off these reviews, written by large teams of busy and opinionated individuals.
For ours, we are greatly in debt to Tom Markland for his perseverance and leadership.
We welcome any comments about the contents of the review.
Wednesday, April 6, 2016
Codifying systematic trends in d- and f-electron materials
As one moves around the periodic table the extent of delocalisation of valence electrons varies significantly and in a systematic way, particularly in transition metals and rare earths. This leads to a subtle competition between metallic and magnetic behaviour and is nicely codified in a reorganised periodic table presented by Smith and Kmetko in 1983.
The version below is presented by Piers Coleman in his long-awaited textbook Introduction to Many-Body Physics.
The bottom part illustrates how as one moves from 3d to 5f to 4f the electrons become more localised due to the tail of the atomic wave function becoming smaller. As the principle quantum number (n=3,4,5) increases the electrons become more delocalised to the larger number of nodes in the radial wave function.
Materials near the localisation-delocalisation boundary are both the most interesting and the most challenging to describe theoretically.
Note that plutonium is at the boundary, which is arguably why it has such a rich phase diagram and properties characteristic of a strongly correlated metal.
The version below is presented by Piers Coleman in his long-awaited textbook Introduction to Many-Body Physics.
The bottom part illustrates how as one moves from 3d to 5f to 4f the electrons become more localised due to the tail of the atomic wave function becoming smaller. As the principle quantum number (n=3,4,5) increases the electrons become more delocalised to the larger number of nodes in the radial wave function.
Materials near the localisation-delocalisation boundary are both the most interesting and the most challenging to describe theoretically.
Note that plutonium is at the boundary, which is arguably why it has such a rich phase diagram and properties characteristic of a strongly correlated metal.
Monday, April 4, 2016
Why I don't worry about getting scooped
Some witty colleagues might say it is because I am not doing anything worth scooping!
Some scientists live in a constant fear of being scooped, i.e. not being the first to publish their latest research result. This can lead to people being very secretive about what they are working on and/or being in an incredible rush to publish. Some groups even require members to sign confidentiality agreements about talking to outsiders.
I always find it strange and disappointing when I ask someone what they are working on and then at some point they say, "I can't say anymore because I don't want to be scooped."
In most cases this strikes me as both egotistical and unrealistic. Most of what we are working on is not so important that others are going to drop everything they are currently doing, steal our idea, work out all the details, and rush to publish...
Sorry to disillusion you.
I often write on this blog about things I am currently working on, long before I have a preprint.
Someone once suggested to me that this was a bit "gutsy" or risky.
To be honest, I would be flattered if someone read a post here and "stole my ideas".
I also don't worry about getting scooped because I have had several concrete "scooped" experiences that showed me that in the long term it did not really matter, particularly in terms of publications.
The stories below show how things are all a bit random.
1. With some mathematics colleagues I wrote a paper about the exact solution to the BCS Hamiltonian for superconducting nanoparticles. We put the paper on the arXiv and amazingly there was a similar posting of a longer work the same day, just after ours in the daily listing. We sent our paper to PRL, but a referee claimed that we had been "beaten" by the other paper! Our paper ended up in PRB Rapid Communications, the other in PRB.
2. While a postdoc with me, Ben Powell did some nice calculations showing how a RVB solution to a Hubbard-Heisenberg model relevant to organic charge transfer salts gave a d-wave superconducting state. I thought this pretty exciting and that we should write the results up for PRL. However, even before we started writing two preprints with similar results appeared on the arXiv. I suggested we write the paper as quick as possible, put it on the arXiv, and send it to PRL anyway. We did and our paper did make it into PRL, along with the other two!
3. Another postdoc completed a draft paper with me, that I then sent to a colleague for comment. He informed me that he read the abstract and realised his postdoc had similar results. He said he would not read more and requested that we both submit to the arXiv on the same day and then send the two papers to PRL, acknowledging each others existence. I agreed. Neither paper made it into PRL. Ours ended up in PRB Rapid Communications. For some reason the other paper was delayed for more than a year.
4. Another postdoc visited his home country and gave talks about our work. Later it was brought to my attention that someone who he had talked to wrote a similar paper, with no acknowledgement of our preprint. When challenged the author claimed it was all different and there was no need to acknowledge our paper.
5. I once gave a talk at which I discussed work that had just been published. A year later someone in the audience published a very similar paper with no acknowledgement of the published work I had described.
So chill out!
It is probably not worth worrying out.
Get some perspective. Remember that in academia the stakes are low.
The cartoon by John R. McKiernan is from here and features in this blog post.
Do you have any experiences of being scooped?
Did it really matter in the end?
Some scientists live in a constant fear of being scooped, i.e. not being the first to publish their latest research result. This can lead to people being very secretive about what they are working on and/or being in an incredible rush to publish. Some groups even require members to sign confidentiality agreements about talking to outsiders.
I always find it strange and disappointing when I ask someone what they are working on and then at some point they say, "I can't say anymore because I don't want to be scooped."
In most cases this strikes me as both egotistical and unrealistic. Most of what we are working on is not so important that others are going to drop everything they are currently doing, steal our idea, work out all the details, and rush to publish...
Sorry to disillusion you.
I often write on this blog about things I am currently working on, long before I have a preprint.
Someone once suggested to me that this was a bit "gutsy" or risky.
To be honest, I would be flattered if someone read a post here and "stole my ideas".
I also don't worry about getting scooped because I have had several concrete "scooped" experiences that showed me that in the long term it did not really matter, particularly in terms of publications.
The stories below show how things are all a bit random.
1. With some mathematics colleagues I wrote a paper about the exact solution to the BCS Hamiltonian for superconducting nanoparticles. We put the paper on the arXiv and amazingly there was a similar posting of a longer work the same day, just after ours in the daily listing. We sent our paper to PRL, but a referee claimed that we had been "beaten" by the other paper! Our paper ended up in PRB Rapid Communications, the other in PRB.
2. While a postdoc with me, Ben Powell did some nice calculations showing how a RVB solution to a Hubbard-Heisenberg model relevant to organic charge transfer salts gave a d-wave superconducting state. I thought this pretty exciting and that we should write the results up for PRL. However, even before we started writing two preprints with similar results appeared on the arXiv. I suggested we write the paper as quick as possible, put it on the arXiv, and send it to PRL anyway. We did and our paper did make it into PRL, along with the other two!
3. Another postdoc completed a draft paper with me, that I then sent to a colleague for comment. He informed me that he read the abstract and realised his postdoc had similar results. He said he would not read more and requested that we both submit to the arXiv on the same day and then send the two papers to PRL, acknowledging each others existence. I agreed. Neither paper made it into PRL. Ours ended up in PRB Rapid Communications. For some reason the other paper was delayed for more than a year.
4. Another postdoc visited his home country and gave talks about our work. Later it was brought to my attention that someone who he had talked to wrote a similar paper, with no acknowledgement of our preprint. When challenged the author claimed it was all different and there was no need to acknowledge our paper.
5. I once gave a talk at which I discussed work that had just been published. A year later someone in the audience published a very similar paper with no acknowledgement of the published work I had described.
So chill out!
It is probably not worth worrying out.
Get some perspective. Remember that in academia the stakes are low.
The cartoon by John R. McKiernan is from here and features in this blog post.
Do you have any experiences of being scooped?
Did it really matter in the end?
Friday, April 1, 2016
Insightful graphs from The Economist
Each week I read The Economist. Many of their articles feature graphs of social or economic data. To me some of them are just random noise. But others are quite dramatic or insightful. Previously, I posted a famous one about smoking.
Below I show two graphs that I thought were quite useful about universities.
This is from an article Brains without borders
This is from an article about how university students often unfairly evaluate their lecturers.
Below I show two graphs that I thought were quite useful about universities.
This is from an article Brains without borders
This is from an article about how university students often unfairly evaluate their lecturers.
Subscribe to:
Comments (Atom)
A golden age for precision observational cosmology
Yin-Zhe Ma gave a nice physics colloquium at UQ last week, A Golden Age for Cosmology I learnt a lot. Too often, colloquia are too speciali...

-
This week Nobel Prizes will be announced. I have not done predictions since 2020 . This is a fun exercise. It is also good to reflect on w...
-
Is it something to do with breakdown of the Born-Oppenheimer approximation? In molecular spectroscopy you occasionally hear this term thro...
-
Nitrogen fluoride (NF) seems like a very simple molecule and you would think it would very well understood, particularly as it is small enou...