In Seattle, I had a really interesting and helpful discussion with Charlie Campbell about doped rare earth oxides.
Cerium oxides have attracted a lot of industrial attention because they have an amazing ability to reversibly release and uptake oxygen. [Just like hemoglobin in your blood!]. Hence, along with many others I thought this was a fundamental issue about pure cerium oxide. However, it turns out all the industrial materials (such as solid oxide fuel cells) are doped with transition metal ions. So the fundamental problem is the following: mixed alloys of ceria and zirconia (ZrO2) have this large uptake-release capacity; it is much larger than pure zirconia or pure ceria.
This paper [which my Indian colleagues made me aware of when I visited Bangalore earlier this year] examines the corresponding question for titania-ceria alloys. [A paper on zirconia-ceria is here.] They find that in the alloys there is a significant relaxation of the oxygen sublattice. In particular four of the metal-oxygen bonds become much longer, reflecting weak bonding of oxygen.
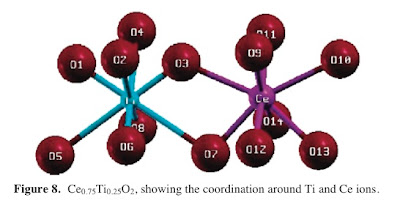 I wonder whether
I wonder whether -thinking about a Jahn-Teller distortion could be helpful here?
-there are high resolution crystal structure data that is amenable to the bond valence sum analysis similar to that performed here.


No comments:
Post a Comment